5,6-二甲基-2,1,3-苯并硒酸二唑 | 2626-34-8
中文名称
5,6-二甲基-2,1,3-苯并硒酸二唑
中文别名
——
英文名称
5,6-dimethyl-2,1,3-benzoselenadiazole
英文别名
5,6-Dimethyl-benzo-2,1,3-selenadiazol;5,6-Dimethyl-2,1,3-benzoselenadiazol
CAS
2626-34-8
化学式
C8H8N2Se
mdl
——
分子量
211.125
InChiKey
JXAGKGMQYSONPB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:143-144 °C
-
沸点:291.4±43.0 °C(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2902909090
SDS
上下游信息
反应信息
-
作为反应物:描述:5,6-二甲基-2,1,3-苯并硒酸二唑 在 sodium nitrate 、 硫酸 作用下, 反应 0.08h, 生成 4,7-dinitro-5,6-dimethylbenzo-2,1,3-selenadiazole参考文献:名称:Sergeev, V. A.; Pesin, V. G.; Papirnik, M. P., Journal of Organic Chemistry USSR (English Translation), 1989, vol. 25, # 8.2, p. 1629 - 1630摘要:DOI:
-
作为产物:描述:4,5-二甲基-1,2-苯二胺 在 selenium(IV) oxide 作用下, 生成 5,6-二甲基-2,1,3-苯并硒酸二唑参考文献:名称:苯并与取代的2,1,3-苯并硒基二唑加合物的形成和重排摘要:通过将苯炔加到取代的2,1,3-苯并硒基二唑上,已制备了一系列5-(1,2-苯并硒唑-3-基)戊二烯腈衍生物(2)。这些加合物中的一些通过热或光化学重排得到2-(2-吡啶基)苯基硒氰酸酯(7),将其还原为2-苯基吡啶衍生物(6)或水解得到最终的二硒化物(9)。报告了一种苯并加合物(2b)的晶体结构,并讨论了其重排机理。DOI:10.1039/p19810000607
文献信息
-
Oxidationsreaktionen methyl-substituierter 2,1,3-Benzothiadiazole und 2,1,3-Benzoselenadiazole mit Selen-dioxid作者:Richard Neidlein、Dagmar KnechtDOI:10.1002/hlca.19870700411日期:1987.7.8Oxidation Reactions of Methyl Substituted 2,1,3-Benzothiadiazoles and 2,1,3-Benzoselenadiazoles with Selenium Dioxide
-
A Paternò–Büchi Reaction of Aromatics with Quinones under Visible Light Irradiation作者:Wen-Wen Li、Jia-Lin Zhao、Ze-Yu Wang、Pei-Ting Li、Zi-Fa Shi、Xiao-Ping Cao、Qiang LiuDOI:10.3390/molecules29071513日期:——Paternò–Büchi reaction of aromatic double bonds with quinones under visible light irradiation. The reactions of aromatics with quinones exposed to blue LED irradiation yielded oxetanes at −78 °C, which was attributed to both the activation of double bonds in aromatics and the stabilization of oxetanes by thiadiazole, oxadiazole, or selenadiazole groups. The addition of Cu(OTf)2 to the reaction system at
-
Hassan, Attard F.; Radhy, Hanan A.; Zayed, Egyptian Journal of Chemistry, 2008, vol. 51, # 4, p. 477 - 484作者:Hassan, Attard F.、Radhy, Hanan A.、ZayedDOI:——日期:——
-
Investigations of 2,1,3-thia- and selenadiazole s作者:V. A. Sergeev、V. G. Pesin、N. M. KotikovaDOI:10.1007/bf00475285日期:1972.3
-
Investigations in the field of 2, 1,3-thiadiazole and 2, 1,3-selenadiazole作者:V. G. Pesin、V. A. Sergeev、A. G. NesterovaDOI:10.1007/bf00478084日期:——
表征谱图
-
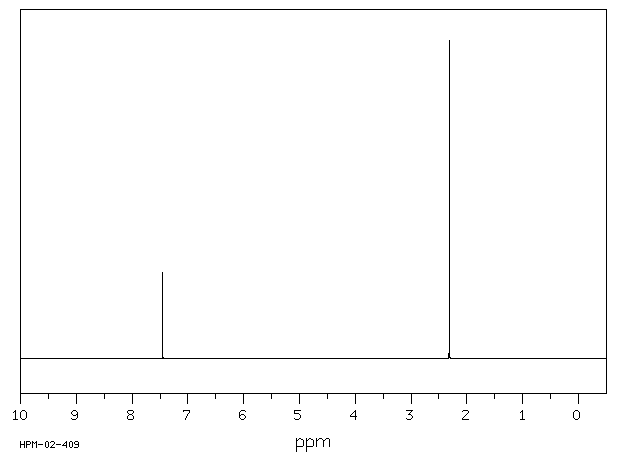
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
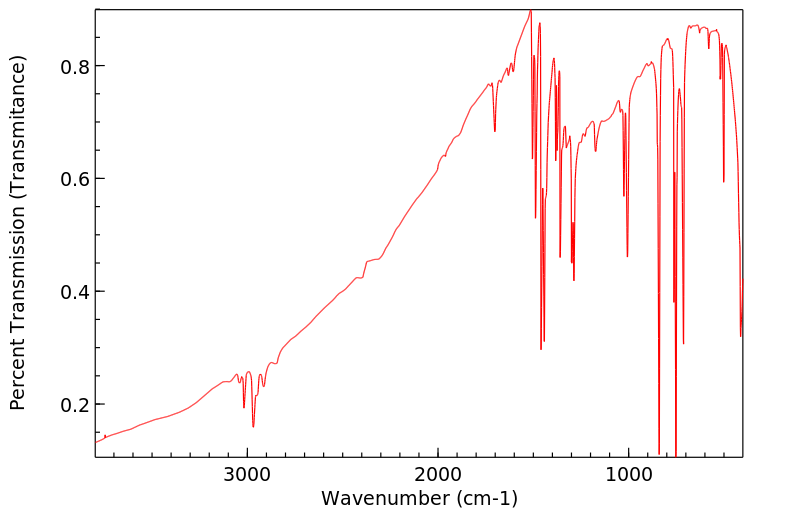
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-(+)-5,5'',6,6'',7,7'',8,8''-八氢-3,3''-二叔丁基-1,1''-二-2-萘酚,双钾盐
(S)-盐酸沙丁胺醇
(S)-溴烯醇内酯
(S)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2-N-Fmoc-氨基甲基吡咯烷盐酸盐
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(6,6)-苯基-C61己酸甲酯
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,5R)-3,3a,8,8a-四氢茚并[1,2-d]-1,2,3-氧杂噻唑-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aS,8aR)-2-(吡啶-2-基)-8,8a-二氢-3aH-茚并[1,2-d]恶唑
(3aS,3''aS,8aR,8''aR)-2,2''-环戊二烯双[3a,8a-二氢-8H-茚并[1,2-d]恶唑]
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(3-三苯基甲氨基甲基)吡啶
(3-[(E)-1-氰基-2-乙氧基-2-hydroxyethenyl]-1-氧代-1H-茚-2-甲酰胺)
(2′′-甲基氨基-1,1′′-联苯-2-基)甲烷磺酰基铝(II)二聚体
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,4S)-Fmoc-4-三氟甲基吡咯烷-2-羧酸
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环