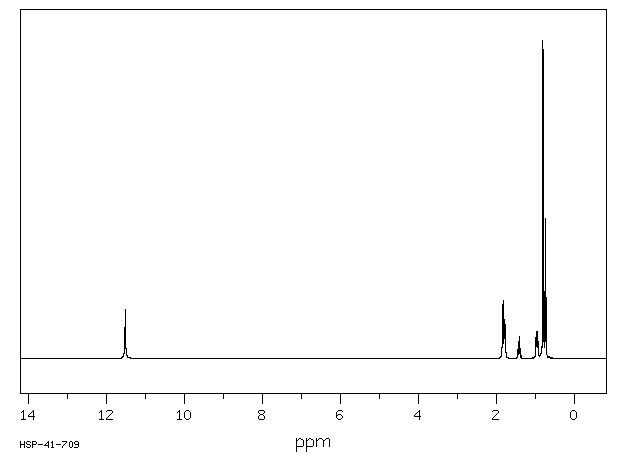
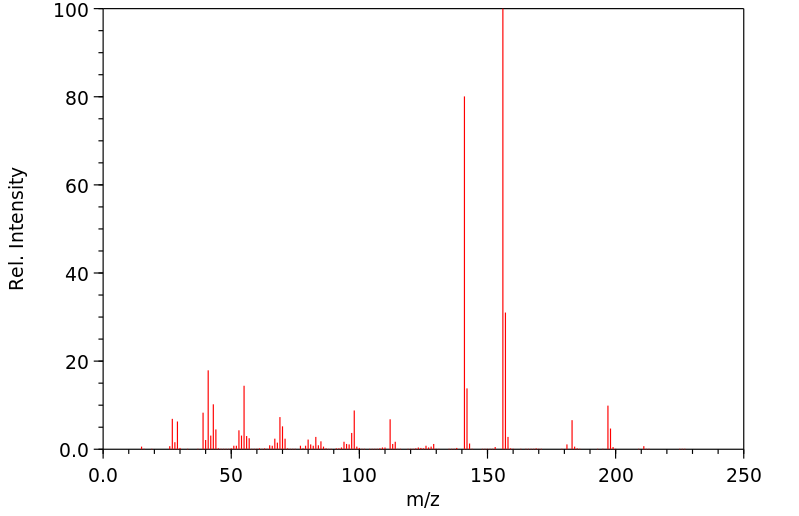
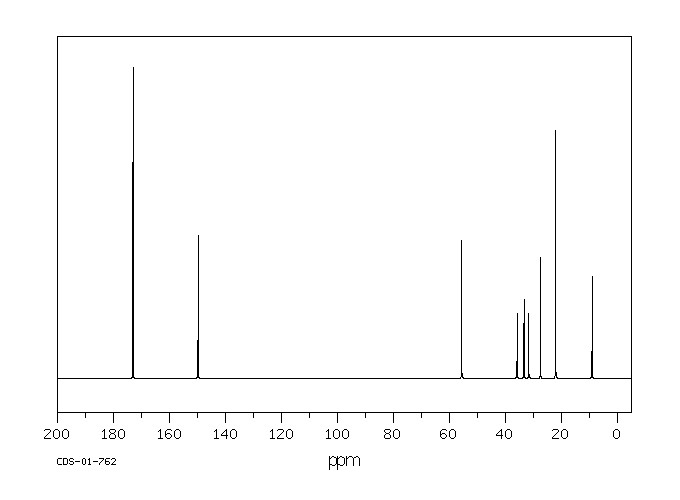
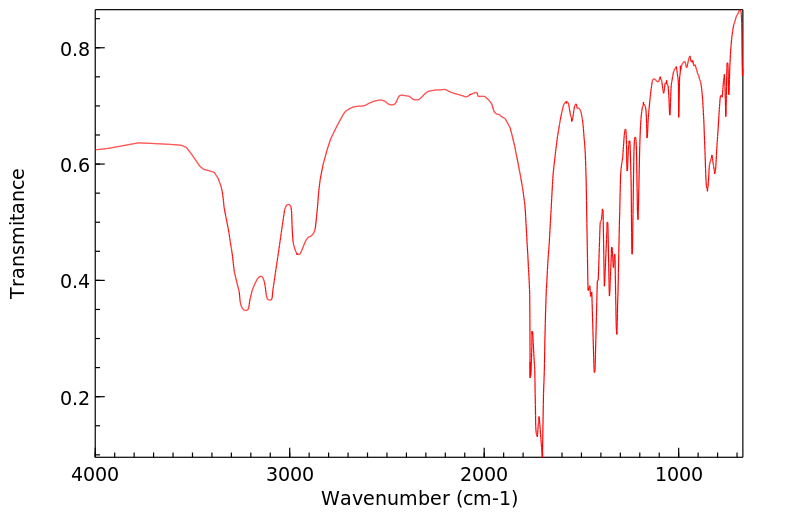
代谢
戊巴比妥通过肝脏代谢,通过3-甲基丁基基团的次末端氧化形成三级醇,羟基戊巴比妥,这是一种无活性的代谢物。口服催眠剂量的大约40-50%的戊巴比妥以羟基戊巴比妥及其葡萄糖醛酸苷形式从尿液中排出。不到1%的口服催眠剂量的药物未改变地从尿液中排出。粪便或尿液中排出的羟基戊巴比妥的苷结合物和/或尚未确定的进一步氧化产物可能占剩余剂量的部分。
Amobarbital is metabolized by the liver via penultimate oxidation of the 3-methylbutyl substituent to form a tertiary alcohol, hydroxyamobarbital, which is an inactive metabolite. About 40-50% of an oral hypnotic dose of amobarbital is excreted in urine as hydroxyamobarbital and its glucuronide conjugates. Less than 1% of an oral hypnotic dose of the drug is excreted in urine unchanged. Conjugates of hydroxyamobarbital excreted in feces or urine and/or further oxidation products not yet identified may account for the remainder of the dose.
来源:Hazardous Substances Data Bank (HSDB)