二乙基硫代膦酰-二乙基-硫代膦烷 | 3790-23-6
中文名称
二乙基硫代膦酰-二乙基-硫代膦烷
中文别名
——
英文名称
tetraethyldiphosphine disulfide
英文别名
Tetraaethyl diphosphine disulphide;Tetraethyldiphosphindisulfid;Tetraaethyl-diphosphin-disulfid;Tetraethyldiphosphine disulphide;diethylphosphinothioyl-diethyl-sulfanylidene-λ5-phosphane
CAS
3790-23-6
化学式
C8H20P2S2
mdl
——
分子量
242.326
InChiKey
LNMKVDPXBCIMOU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:12
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:64.2
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2930909090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— tetraethyldiphosphane monosulfide —— C8H20P2S 210.26
反应信息
-
作为反应物:描述:二乙基硫代膦酰-二乙基-硫代膦烷 在 三氟化锑 作用下, 反应 2.0h, 以52.5 g的产率得到Diethyl-trifluor-phosphoran参考文献:名称:一种次膦酸酯的制备方法、次膦酸酯及非水电 解液摘要:本发明提供一种次膦酸酯的制备方法、次膦酸酯及其非水电解液。所述一种次膦酸酯的制备方法,包括以下步骤:(1)通过格氏试剂R1MgX与三卤硫磷X3P=S在溶剂中反应,得到第一中间体;(2)通过卤化剂M’Xa与所述第一中间体进行反应,得到第二中间体;(3)通过R2OH、水与所述第二中间体反应,得到次膦酸酯;其中,R1、R2分别选自烃基、或含有硼、硅、氮、磷、氧、硫、氟、氯、溴及碘中至少一种元素的有机基团;X表示卤素;M’表示金属元素;a表示金属元素M’的化合价。使用本发明次膦酸酯制备的电解液可以提升二次电池的高温循环稳定性;同时还具有不燃、阻燃或自熄特性,可以提升二次的电池安全性能。公开号:CN108017669B
-
作为产物:参考文献:名称:一种次膦酸酯的制备方法、次膦酸酯及非水电 解液摘要:本发明提供一种次膦酸酯的制备方法、次膦酸酯及其非水电解液。所述一种次膦酸酯的制备方法,包括以下步骤:(1)通过格氏试剂R1MgX与三卤硫磷X3P=S在溶剂中反应,得到第一中间体;(2)通过卤化剂M’Xa与所述第一中间体进行反应,得到第二中间体;(3)通过R2OH、水与所述第二中间体反应,得到次膦酸酯;其中,R1、R2分别选自烃基、或含有硼、硅、氮、磷、氧、硫、氟、氯、溴及碘中至少一种元素的有机基团;X表示卤素;M’表示金属元素;a表示金属元素M’的化合价。使用本发明次膦酸酯制备的电解液可以提升二次电池的高温循环稳定性;同时还具有不燃、阻燃或自熄特性,可以提升二次的电池安全性能。公开号:CN108017669B
-
作为试剂:描述:甲基二氯硅烷 、 1-庚烯 在 二乙基硫代膦酰-二乙基-硫代膦烷 、 nickel dichloride 作用下, 反应 10.0h, 生成 庚基甲基二氯硅烷 、 chloroheptylmethylsilane参考文献:名称:Kalinin, A. V.; Trofimov, A. E.; Skvortsov, N. K., Russian Journal of General Chemistry, 1997, vol. 67, # 5, p. 820摘要:DOI:
文献信息
-
Equilibrium shift in the rhodium-catalyzed acyl transfer reactions作者:Mieko Arisawa、Yui Igarashi、Haruki Kobayashi、Toru Yamada、Kentaro Bando、Takuya Ichikawa、Masahiko YamaguchiDOI:10.1016/j.tet.2011.07.031日期:2011.10enzene. Acid fluorides were converted into acylphosphine sulfides and thioesters using diphosphine disulfides and disulfides/triphenylphosphine, respectively. Aryl esters were obtained from acid fluorides and phenols in the presence of triphenylsilane. Aryl esters, acylphosphine sulfides, and thioesters were also interconverted in the presence of rhodium complexes. These rhodium-catalyzed acyl transfer
-
Structure and bonding in gold(<scp>I</scp>) compounds. Part 3. Mössbauer spectra of three-co-ordinate complexes作者:Geoffrey C. H. Jones、Peter G. Jones、Alfred G. Maddock、Martin J. Mays、Pauline A. Vergnano、Alan F. WilliamsDOI:10.1039/dt9770001440日期:——Mössbauer spectra are reported for a number of gold(I) complexes which might be expected to be three-co-ordinate. The spectral data are related to the possible electronic and geometric structures of these complexes. It is shown that the Mössbauer spectrum provides a useful, although not invariably unambiguous, indication of geometry.报告了许多金(I)配合物的Mössbauer光谱,这些配合物可能期望是三坐标的。光谱数据与这些配合物的可能的电子和几何结构有关。结果表明,穆斯堡尔光谱提供了有用的,但并非总是明确的几何图形。
-
Rhodium-catalyzed P–P bond exchange reaction of diphosphine disulfides作者:Mieko Arisawa、Tomoki Yamada、Saori Tanii、Yuta Kawada、Hisako Hashimoto、Masahiko YamaguchiDOI:10.1039/c6cc07302f日期:——A rhodium-catalyzed exchange reaction of diphosphine disulfides, a diphosphine oxide, and a diphosphine is developed. Various symmetric diphosphine disulfides containing alkyl and phenyl groups are exchanged, giving equilibrium mixtures, from...
-
Rhodium-Catalyzed Synthesis of Dialkyl(Heteroaryl)Phosphine Sulfides by Phosphinylation of Heteroaryl Sulfides作者:Mieko Arisawa、Takeru Tazawa、Wataru Ichinose、Haruki Kobayashi、Masahiko YamaguchiDOI:10.1002/adsc.201800630日期:2018.9.17Dialkyl(heteroaryl)phosphine sulfides were synthesized by the rhodium‐catalyzed exchange reaction of heteroaryl aryl sulfides and tetraalkyldiphosphine disulfides. The reaction has a broad applicability, giving diverse dialkyl(heteroaryl)phosphine sulfides containing five‐ and six‐membered heteroaryl groups. Various dialkyl(heteroaryl)phosphines were obtained by desulfurization.
-
Metal-catalyzed phosphinyl ester forming reaction of alcohols and phenols with diphosphine disulfides and a dioxide作者:Mieko Arisawa、Masahiko YamaguchiDOI:10.1016/j.tetlet.2010.07.040日期:2010.9the dialkylphosphinothioation reaction of alcohols and phenols with tetraalkyldiphosphine disulfides in high yields. Phenols were reacted in the presence of RhH(PPh3)4 and 1,2-bis(dimethylphosphino)ethane under THF reflux, and alcohols with Pd(OAc)2 and 1,2-bis(diphenylphosphino)benzene under chlorobenzene reflux. Primary alcohols reacted faster than secondary alcohols under these conditions, and protected
表征谱图
-
氢谱1HNMR
-
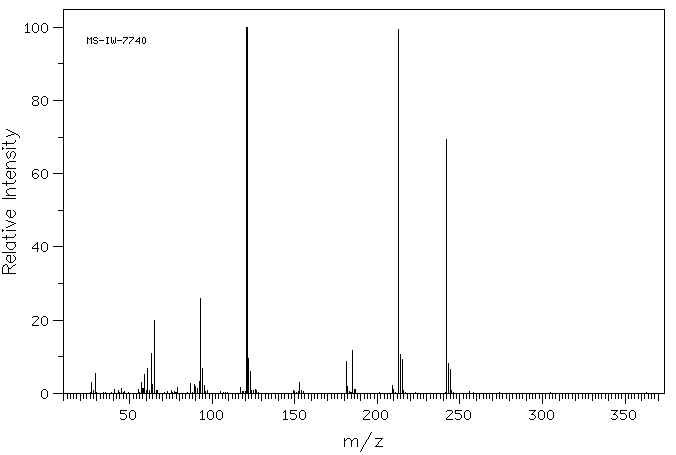
质谱MS
-
碳谱13CNMR
-
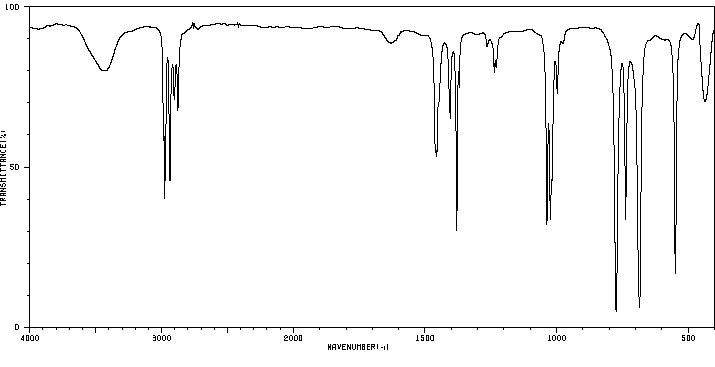
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿米福汀二钠
钠二乙基硫代亚膦酸酯
钠二丁基膦基二硫代酸酯
膦基硫杂酰胺,N-[二(1-甲基乙基)硫膦基]-P,P-二(1-甲基乙基)-
膦二氯化,[1,2-二氯-2-[(1-甲基乙基)硫代]乙烯基]-,(E)-
脱叶磷
脱叶亚磷
磷羧基硫酸,甲基-,S-丁基O-庚基酯(8CI,9CI)
磷羧基硫酸,甲基-,S-丁基O-己基酯(8CI,9CI)
磷氰酸根硫杂二酰胺(9CI)
硫线磷
硫代磷酸二氢S-(2-氨基-2-甲基丙基)酯
硫代磷酸二氢 S-(3-氨基丙基)酯
硫代磷酸三(2-乙基己基)酯
硫代磷酸S-[2-[[3-(乙基氨基)丙基]氨基]乙基]酯
硫代磷酸S-[2-(二乙氧基亚膦酰氨基)乙基]O,O-二乙基酯
硫代磷酸S-[(1-氨基环戊基)甲基]酯
硫代磷酸S-(4-氯-2-丁烯-1-基)O,O-二乙酯
硫代磷酸S-(2,2-二氯乙烯基)O,O-二乙酯
硫代磷酸O-(2-甲氧基乙基)O-甲基S-(2-丙炔基)酯
硫代磷酸O-(2-乙氧基乙基)O-甲基S-(2-丙炔基)酯
硫代磷酸O,O-二甲基S-(2,2,2-三氯乙基)酯
硫代磷酸O,O-二乙基S-(3,4,4-三氟-3-丁烯基)酯
硫代磷酸O,O-二乙基S-(1,2,2-三氯乙基)酯
硫代磷酸3-((2-氨基乙基)氨基)丙硫醇S-酯
硫代磷酸,S-(1,1-二甲基乙基)O,O-二乙酯
硫代磷酸 O,S-二甲基酯钠盐
甲胺磷
甲胺磷
甲硫基膦酸 O,S-二甲基酯
甲硫基膦酸 O,O-二甲酯
甲氧基(甲基硫烷基)次膦酸
甲拌酯
甲基硫代膦酸
甲基硫代磷酸二乙酯
甲基硫代磷酰氯
甲基内吸磷
甲基二硫代膦酸二丙酯
甲基二硫代膦酸 S,S-二丙酯
甲基二硫代氯膦酸O-丁酯
甲基三硫代膦酸二丙酯
环戊烯基硫代磷酸酯
灭线磷
氯甲基硫膦
氨磷汀三水合物
氨磷汀一水物
氨磷汀
氧甲拌磷
正丙基二氯硫膦
果虫磷