(E)-cinnamyl phenylacetate | 133871-04-2
中文名称
——
中文别名
——
英文名称
(E)-cinnamyl phenylacetate
英文别名
cinnamyl 2-phenylacetate;Cinnamyl phenylacetate;[(E)-3-phenylprop-2-enyl] 2-phenylacetate
CAS
133871-04-2
化学式
C17H16O2
mdl
——
分子量
252.313
InChiKey
SFXQCOMMEMBETJ-KPKJPENVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
物理描述:Colourless, slightly viscous liquid, deep chrysanthemum-like odour
-
溶解度:insoluble in water
-
密度:1.089-1.095
-
折光率:1.575-1.581
-
保留指数:1820;1820.4
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:19
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:(E)-cinnamyl phenylacetate 在 Montmorillonite K-10 clay 、 苯甲醚 作用下, 反应 0.33h, 以85%的产率得到苯乙酸参考文献:名称:Microwave accelerated selective and facile deprotection of allyl esters catalyzed by Montmorillonite K-10摘要:在无溶剂条件下,使用微波辐照蒙脱石 K-10 从相应的取代烯丙基酯中再生羧酸,与热条件相比,产量提高,反应时间缩短。DOI:10.1039/a909265j
-
作为产物:描述:(E)-1-(2-(cinnamyloxy)-2-oxoethyl)tetrahydro-1H-thiophen-1-ium tetraphenylborate 以60%的产率得到(E)-cinnamyl phenylacetate参考文献:名称:四苯基硼酸sulf离子对之间的无金属碳-碳交叉偶联摘要:通过将卤化with与四苯基硼酸钠复分解,可以容易地制备一系列四苯基硼酸bor。在120–150°C加热后,四苯基硼酸sulf可以在没有金属催化剂的情况下平稳地经历四苯基硼酸根阴离子和sulf阳离子之间的交叉偶联。对于羰基甲基,苄基和烯丙基硫,相应的羰基甲基-苯基,苄基-苯基和烯丙基-苯基交叉偶联产物的收率可达22-76%。提出了用于该交叉偶联反应的离子间电子转移机理。DOI:10.1016/j.tetlet.2014.05.102
文献信息
-
Benzyne-Mediated Esterification Reaction作者:Jinlong Zhao、Jiarong Shi、Yang LiDOI:10.1021/acs.orglett.1c02702日期:2021.9.17A benzyne-mediated esterification of carboxylic acids and alcohols under mild conditions has been realized, which is made possible via a selective nucleophilic addition of carboxylic acid to benzyne in the presence of alcohol. After a subsequent transesterification with alcohol, the corresponding esters can be produced efficiently. This benzyne-mediated protocol can be used on the modification of Ibuprofen
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
Aerobic Acyloxylation of Allylic C−H Bonds Initiated by a Pd <sup>0</sup> Precatalyst with 4,5‐Diazafluoren‐9‐one as an Ancillary Ligand作者:Caitlin V. Kozack、Jennifer A. Sowin、Jonathan N. Jaworski、Andrei V. Iosub、Shannon S. StahlDOI:10.1002/cssc.201900727日期:2019.7.5Palladium‐catalyzed allylic C−H oxidation has been widely studied, but most precedents use acetic acid as the coupling partner. In this study, a method compatible with diverse carboxylic acid partners has been developed. Use of a Pd0 precatalyst under aerobic reaction conditions leads to oxidation of Pd0 by O2 in the presence of the desired carboxylic acid to generate a PdII dicarboxylate that promotes
-
Pd-Catalyzed Carbonylation of Diazo Compounds at Atmospheric Pressure: A Catalytic Approach to Ketenes作者:Zhenhua Zhang、Yiyang Liu、Lin Ling、Yuxue Li、Yian Dong、Mingxing Gong、Xiaokun Zhao、Yan Zhang、Jianbo WangDOI:10.1021/ja107351d日期:2011.3.30and affects the diastereoselectivity of the β-lactam products by assisting isomerization of the addition intermediate. On the other hand, the acylketenes generated from the Pd-catalyzed carbonylation of α-diazoketones react with imines in a formal [4 + 2] cycloaddition manner to afford 1,3-dioxin-4-one derivatives. This straightforward carbonylation provides a new approach toward highly efficient catalytic由于乙烯酮介导的反应在有机合成中的重要性,因此非常需要通过催化循环使卡宾羰基化。在这项研究中,基于 Pd 催化重氮化合物与 CO 的羰基化,开发了一种高效、温和的乙烯酮中间体催化方法。 当 α-重氮羰基化合物或 N-甲苯磺酰腙盐在钯催化剂存在下在常压下加热时在 CO 压力下,烯酮中间体在原位形成,在那里它们与各种亲核试剂(如醇、胺或亚胺)进行进一步反应。Pd 催化的串联羰基化-施陶丁格环加成反应以良好的产率得到 β-内酰胺衍生物,并具有优异的反式非对映选择性。反应机理的 DFT 计算结果表明,Pd 参与 [2+2] 环加成过程,并通过辅助加成中间体的异构化影响 β-内酰胺产物的非对映选择性。另一方面,由 Pd 催化的 α-重氮酮羰基化产生的酰基烯酮与亚胺以正式的 [4 + 2] 环加成方式反应,得到 1,3-dioxin-4-one 衍生物。这种直接的羰基化提供了一种在温和条件下高效催化生成烯酮物种的新方法。由
-
Tandem Insertion/[3,3]-Sigmatropic Rearrangement Involving the Formation of Silyl Ketene Acetals by Insertion of Rhodium Carbenes into S–Si Bonds作者:Jason R. Combs、Yin-Chu Lai、David L. Van VrankenDOI:10.1021/acs.orglett.1c00229日期:2021.4.16with trimethylsilyl thioethers in the presence of rhodium(II) catalysts to generate α-allyl-α-thio silyl esters. The transformation involves a tandem process involving formal rhodium-catalyzed insertion of the carbene group into the S–Si bond to generate a silyl ketene acetal, followed by a spontaneous Ireland–Claisen rearrangement. The silyl ester products were isolated as the corresponding carboxylic
表征谱图
-
氢谱1HNMR
-
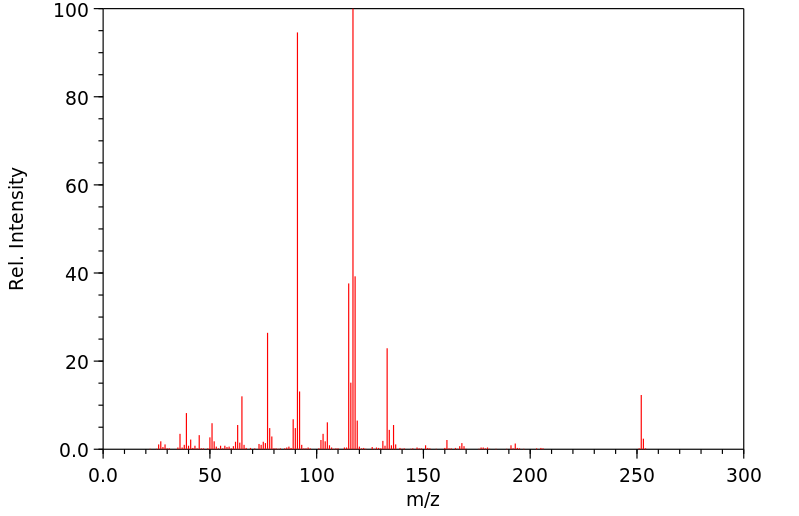
质谱MS
-
碳谱13CNMR
-
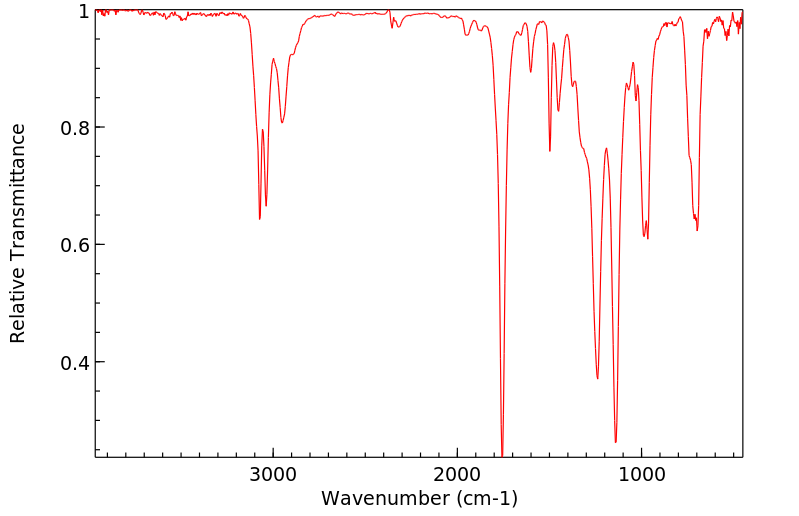
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫