1-哌啶-1-基环己烷甲腈 | 3867-15-0
中文名称
1-哌啶-1-基环己烷甲腈
中文别名
——
英文名称
1-piperidinocyclohexylcarbonitrile
英文别名
1-Piperidinocyclohexanecarbonitrile;1-piperidin-1-ylcyclohexane-1-carbonitrile
CAS
3867-15-0
化学式
C12H20N2
mdl
MFCD00179761
分子量
192.304
InChiKey
WWSAYKJWUZJLRT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:318.3°C (rough estimate)
-
密度:1.0076 (rough estimate)
-
溶解度:DMF:2mg/mL;二甲基亚砜:3mg/mL; DMSO:PBS(pH 7.2)(1:4):0.2 mg/mL;乙醇:1mg/mL
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.92
-
拓扑面积:27
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
海关编码:2933399090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-(1-cyanocyclohexyl)piperidone 121817-69-4 C12H18N2O 206.288 C-(1-哌啶-1-基-环己基)-甲基胺 C-(1-piperidin-1-yl-cyclohexyl)methylamine 41805-36-1 C12H24N2 196.336
反应信息
-
作为反应物:描述:参考文献:名称:Syntheses of amine derivatives of phencyclidine摘要:DOI:10.1021/jo00323a014
-
作为产物:描述:参考文献:名称:Transamination of α-amino nitriles摘要:alpha-Amino nitriles containing a primary amino group undergo transamination with aliphatic and aromatic amines under mild conditions with high yields. A probable reaction mechanism involving intermediate elimination of cyanide ion has been proposed.DOI:10.1134/s1070428014010047
-
作为试剂:描述:3-(2,3,4-trifluorophenyl)-1-[trans-4-(trans-4-propylcyclohexyl)cyclohexyl]-1-propanol 在 1-哌啶-1-基环己烷甲腈 作用下, 以 二氯甲烷 为溶剂, 反应 5.0h, 以94%的产率得到3-(2,3,4-trifluorophenyl)-1-[trans-4-(trans-4-propylcyclohexyl)cyclohexyl]-1-propanone参考文献:名称:JP2005/200366摘要:公开号:
文献信息
-
Synthesis and evaluation of conformationally restricted N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamines at .sigma. receptors. 2. Piperazines, bicyclic amines, bridged bicyclic amines, and miscellaneous compounds作者:Brian R. de Costa、Xiaoshu He、Joannes T. M. Linders、Celia Dominguez、Zi Qiang Gu、Wanda Williams、Wayne BowenDOI:10.1021/jm00068a007日期:1993.84-dichlorophenyl)ethyl]-1,4-diazabicyclo[3.2.2]nonane (16)] show very weak sigma interaction. Comparison of the binding data of different N-substituted homologues of 1 with those of the 1-[2-(3,4-dichlorophenyl)ethyl]-4-alkylpiperazines suggests that the two nitrogen atoms of 1 are working in opposition to one another in terms of their sensitivity to steric bulk. The high binding affinity of the 1,4-diazabicyclo[4作为我们先前研究(J. Med。Chem。1992,35,4334-4343)的继续,我们构象限制了sigma-受体配体2-(1-吡咯烷基)-N- [2-(3,4-二氯苯基)乙基] -N-甲基乙胺(1)掺入一系列同源的哌嗪3-9和均哌嗪10和11中,二氮杂双环壬烷和癸烷,桥头双环辛烷和壬烷以及其他其他化合物。使用[3H](+)-喷他佐辛在豚鼠脑膜sigma 1位点获得sigma-受体结合亲和力。研究表明,在哌嗪中发现的氮孤对取向提供最强的结合相互作用。其他氮孤对取向或代表1 [不可能的交错构象的化合物,如4- [2-(3,4-二氯苯基)乙基] -1,4-二氮杂双环[3.2]。2] nonane(16)]显示非常弱的sigma相互作用。比较1的不同N-取代同系物与1- [2-(3,4-二氯苯基)乙基] -4-烷基哌嗪的结合数据表明,1的两个氮原子彼此相反。就其对空间体积的敏感性而言。1,4-二氮杂双环[4
-
Hypervalent iodine oxidation of amines using iodosobenzene: Synthesis of nitriles, ketones and lactams作者:Robert M Moriarty、Radhe K Vaid、Michael P Duncan、Masahito Ochiai、Minako Inenaga、Yoshimitsu Nagao*DOI:10.1016/s0040-4039(00)88473-7日期:1988.1Primary aliphatic amines on oxidation with iodosobenzene in CH2Cl2 or H2O yield the corresponding nitriles, while primary cycloalkylamines give the corresponding cyclic ketones. Lactams are obtained by the oxidation of cyclic amines. (S)(−) Nicotine () is oxidized to ()-cotinine (). The intermediary imine involved in these processes was trapped in the case of piperidine as the α-aminonitrile.
-
Synthesis and Preliminary Biochemical Evaluation of Novel Derivatives of PCP作者:Joannes Linders、David Furlano、Mariena Mattson、Arthur Jacobson、Kenner RiceDOI:10.2174/157018010790225813日期:2010.2.1(±)-Trans-Ph/Et and (±)-cis-Ph/Et 1-(2-ethyl-1-phenylcyclohexyl)piperidine were synthesized from 2-ethylcyclohexanone. In contrast to the corresponding trans-substituted 2-methyl compound which is 5x more potent than PCP, the trans-2-ethyl derivative has a 75x lower affinity for the PCP binding site. The cis-2-ethyl isomer is inactive like the cis-2-methyl derivative. (±)-1-(1- Phenylcyclohexyl)-2-methylpiperidine is almost as active as the parent PCP. Reduction of the aromatic ring or quaternization of the piperidine in PCP reduces the affinity for the PCP site.
-
Efficient Co(ii) heterogeneously catalysed synthesis of α-aminonitriles at room temperature via Strecker-type reactions作者:Fatemeh Rajabi、Sara Ghiassian、Mohammad Reza SaidiDOI:10.1039/c0gc00047g日期:——An environmentally friendly and highly active mesoporous Co(II) complex on mesoporous SBA-15 material could be used as an easily recoverable catalyst for the synthesis of α-aminonitriles from a wide range of aldehydes/ketones and primary or secondary amines with good to excellent conversions yields at room temperature under solventless conditions. The catalyst can be recovered by simple filtration and could be reused at least 10 times without loss of catalytic activity.一种环境友好且活性高的介孔Co(II)配合物负载于介孔SBA-15材料上,可作为易于回收的催化剂,用于在无溶剂条件下室温下从广泛范围的醛/酮和伯或仲胺合成α-氨基腈,并具有优良至极佳的转化产率。通过简单过滤即可回收该催化剂,并且至少可重复使用10次而不会损失其催化活性。
-
Heterogeneously catalysed Strecker-type reactions using supported Co(ii) catalysts: microwave vs. conventional heating作者:Fatemeh Rajabi、Saghar Nourian、Sara Ghiassian、Alina M. Balu、Mohammad Reza Saidi、Juan Carlos Serrano-Ruiz、Rafael LuqueDOI:10.1039/c1gc15741h日期:——α-aminonitriles could be efficiently prepared from various aldehydes/ketones and primary or secondary amines using a highly active and stable Co(II) complex supported on different mesoporous supports at both room temperature and low temperature microwave irradiation under solventless conditions. Catalysts were also highly reusable under the investigated reaction conditions and could be reused at least 10 times
表征谱图
-
氢谱1HNMR
-
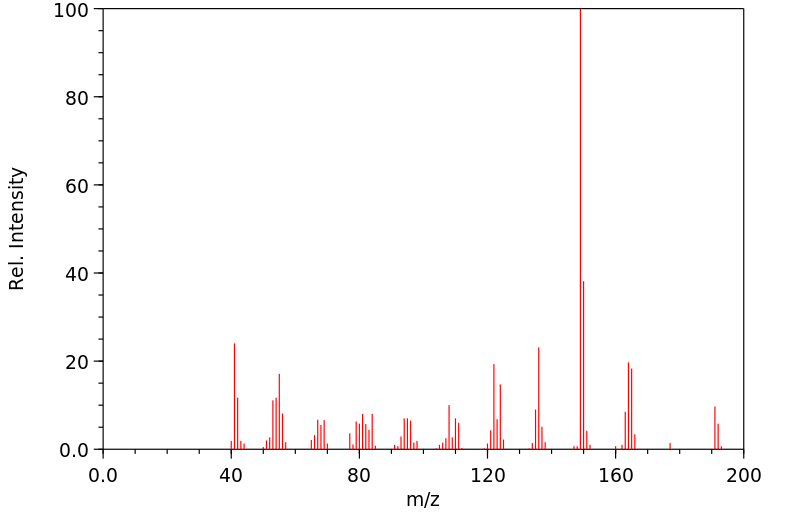
质谱MS
-
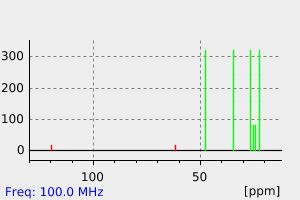
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷