5-[2-(甲硫基)乙基]海因 | 13253-44-6
中文名称
5-[2-(甲硫基)乙基]海因
中文别名
5-[2-(甲硫基)乙基]乙内酰脲;5-[2-甲基硫代乙酰]乙内酰;5-[2-(甲硫)乙基]-2,4-咪唑烷二酮
英文名称
5-[2-(methylthio)ethyl]imidazolidine-2,4-dione
英文别名
5-(2-methylmercaptoethyl)hydantoin;5-(2-methylthioethyl)hydantoin;methionine hydantoin;5-(2-methylsulfanylethyl)imidazolidine-2,4-dione
CAS
13253-44-6
化学式
C6H10N2O2S
mdl
MFCD00143392
分子量
174.224
InChiKey
SBKRXUMXMKBCLD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:108°C
-
密度:1.228±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:83.5
-
氢给体数:2
-
氢受体数:3
安全信息
-
RTECS号:NI9461351
-
海关编码:2933990090
-
安全说明:S22,S24/25
-
储存条件:室温和干燥环境下使用。
SDS
5-[2-(Methylthio)ethyl]hydantoin Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 5-[2-(Methylthio)ethyl]hydantoin
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 5-[2-(Methylthio)ethyl]hydantoin
Percent: >99.0%(T)
CAS Number: 13253-44-6
Chemical Formula: C6H10N2O2S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
5-[2-(Methylthio)ethyl]hydantoin
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:108°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
5-[2-(Methylthio)ethyl]hydantoin
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
orl-rat LDLo:5 g/kg
Acute Toxicity:
skn-rbt LD50:>5 g/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
NI9461351
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
5-[2-(Methylthio)ethyl]hydantoin
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 5-[2-(Methylthio)ethyl]hydantoin
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 5-[2-(Methylthio)ethyl]hydantoin
Percent: >99.0%(T)
CAS Number: 13253-44-6
Chemical Formula: C6H10N2O2S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
5-[2-(Methylthio)ethyl]hydantoin
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:108°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
5-[2-(Methylthio)ethyl]hydantoin
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
orl-rat LDLo:5 g/kg
Acute Toxicity:
skn-rbt LD50:>5 g/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
NI9461351
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
5-[2-(Methylthio)ethyl]hydantoin
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 5-[2-(methylthio)ethyl]-2,4-imidazolidinedione 40856-81-3 C6H10N2O2S 174.224 5-(2-溴乙基)海因 5-(β-bromoethyl)hydantoin 7471-52-5 C5H7BrN2O2 207.027 —— 5-(2-chloro-ethyl)-imidazolidine-2,4-dione —— C5H7ClN2O2 162.576 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,6-二(2-甲硫基乙基)哌嗪-2,5-二酮 methionine diketopiperazine 36963-02-7 C10H18N2O2S2 262.397
反应信息
-
作为反应物:描述:5-[2-(甲硫基)乙基]海因 在 水 、 potassium hydrogencarbonate 、 二氧化碳 作用下, 25.0~180.0 ℃ 、300.01 kPa 条件下, 反应 0.33h, 生成 DL-蛋氨酸参考文献:名称:Method for manufacturing methylmercaptopropionaldehyde and methionine using renewable raw materials摘要:本发明涉及一种制备甲基巯基丙醛(MMP)的方法,至少包括以下步骤:(a)在酸催化剂存在下,脱水甘油以从甘油的水溶液中制备丙烯醛;(b)纯化步骤(a)中的水流,以获得含有至少相对于丙烯醛的水含量为15重量%的丙烯醛流;(c)在催化剂存在下,使步骤(b)中获得的丙烯醛流与甲基巯基反应;(d)可选地纯化步骤(c)中获得的产物。本发明的方法还可以包括将步骤(c)或(d)中获得的产物与氢氰酸或氰化钠在步骤(e)中进行反应,然后进行后续转化以产生蛋氨酸或蛋氨酸羟基类似物,然后可选地纯化。根据本发明的方法中额外使用来自生物质的甲基巯基和/或氢氰酸作为原料,可以获得由可再生来源的100%有机碳组成的MMP、蛋氨酸或蛋氨酸羟基类似物。公开号:US08735631B2
-
作为产物:参考文献:名称:Guivarch, Marcel; Gillonnier, Claude; Brunie, Jean-Claude, Bulletin de la Societe Chimique de France, 1980, vol. 2, # 1-2, p. 91 - 95摘要:DOI:
文献信息
-
[EN] METHOD FOR PREPARING METHIONINE<br/>[FR] PROCÉDÉ DE PRÉPARATION DE MÉTHIONINE申请人:EVONIK DEGUSSA GMBH公开号:WO2018114640A1公开(公告)日:2018-06-28The present invention relates to a method for preparing methionine or methionine salts. In particular, the invention describes the step of preparing 2-hydroxy-4-(methylthio)butyronitrile (MMP-CN) from 3-methylthiopropanal (MMP) and hydrogen cyanide (HCN) in the presence of ammonia by bringing a gaseous mixture comprising HCN and ammonia into contact with MMP.
-
PROCESS FOR PRODUCING METHIONINE申请人:AZEMI Takushi公开号:US20100121104A1公开(公告)日:2010-05-13A process for producing methionine, while corrosion of a pipe and a reaction vessel is well inhibited, is provided including the following steps (1) to (3), wherein a content of thiols in 3-methylthiopropanal is 500 ppm or less, based on the propanal, and a content of hydrogen sulfide in 3-methylthiopropanal is 60 ppm or less, based on the propanal; step (1) in which 3-methylthiopropanal is reacted with hydrogen cyanide in the presence of a base to give 2-hydroxy-4-methylthiobutanenitrile; step (2) in which the 2-hydroxy-4-methylthiobutanenitrile obtained in step (1) is reacted with ammonium carbonate to give 5-(β-methylmercaptoethyl)hydantoin; and step (3) in which the 5-(β-methylmercaptoethyl)hydantoin obtained in step (2) is hydrolyzed in the presence of a basic potassium compound to give methionine.
-
METHOD FOR PRODUCING METHIONINE AND/OR 2-HYDROXY-4-(METHYLTHIO) BUTANOIC ACID申请人:SUMITOMO CHEMICAL COMPANY, LIMITED公开号:US20190077750A1公开(公告)日:2019-03-14An object of the invention is to provide a simple method for producing methionine and/or 2-hydroxy-4-(methylthio)butanoic acid at a high yield using 3-(methylthio)propionaldehyde as a raw material. An oxide catalyst containing cerium, 3-(methylthio)propionaldehyde, a compound containing cyanide ion, ammonia or a compound containing ammonium ion, and water are contacted with each other to produce methionine and/or 2-hydroxy-4-(methylthio)butanoic acid.
-
[EN] METHOD FOR PRODUCING METHIONINE<br/>[FR] PROCÉDÉ DE PRODUCTION DE MÉTHIONINE申请人:EVONIK OPERATIONS GMBH公开号:WO2020120720A1公开(公告)日:2020-06-18The present invention pertains to a method for producing methionine or salts or derivatives thereof from hydrogen cyanide (HCN), the method comprising a step of producing 2-hydroxy-4-(methylthio)butyronitrile (MMP-CN), or a crude product mixture comprising MMP-CN, by contacting a hydrogen cyanide (HCN) process gas mixture prepared according to the Andrussow process from methane, ammonia and oxygen, with 3-methylmercaptopropionaldehyde (MMP), wherein the HCN process gas mixture is obtained from the crude HCN process gas mixture by adjusting the amount of ammonia to between 20 % (v/v) and 60% (v/v) of the amount of the ammonia in the crude HCN process gas mixture.
-
METHOD FOR PRODUCING METHIONINE申请人:SUMITOMO CHEMICAL COMPANY, LIMITED公开号:US20210070704A1公开(公告)日:2021-03-11An object of the present invention to provide a method for producing methionine with consideration given to the environment. The production method of the present invention comprises a removal step of blowing an inert gas into a liquid containing 5-(2-methylmercaptoethyl)hydantoin and thereby diffusing ammonia remaining in the liquid to obtain an emission gas containing the ammonia, and a recovery step of bringing the emission gas into contact with a washing liquid to recover ammonia contained in the emission gas.
表征谱图
-
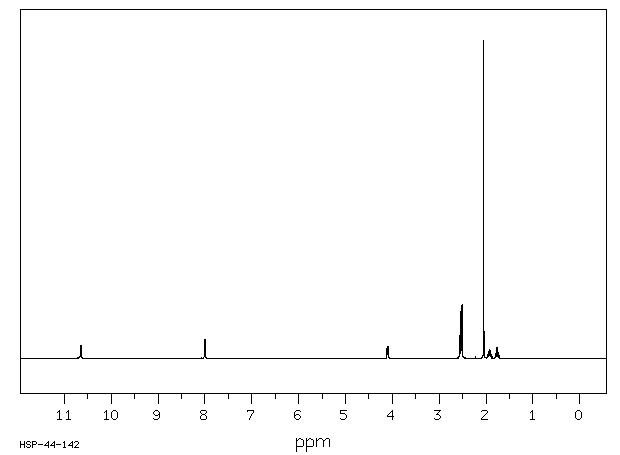
氢谱1HNMR
-
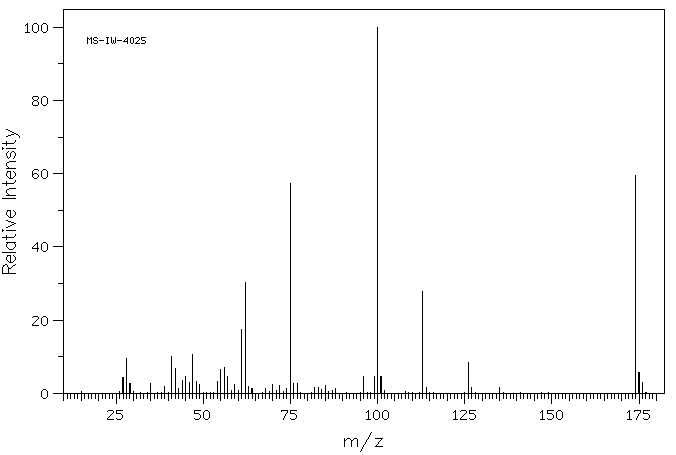
质谱MS
-
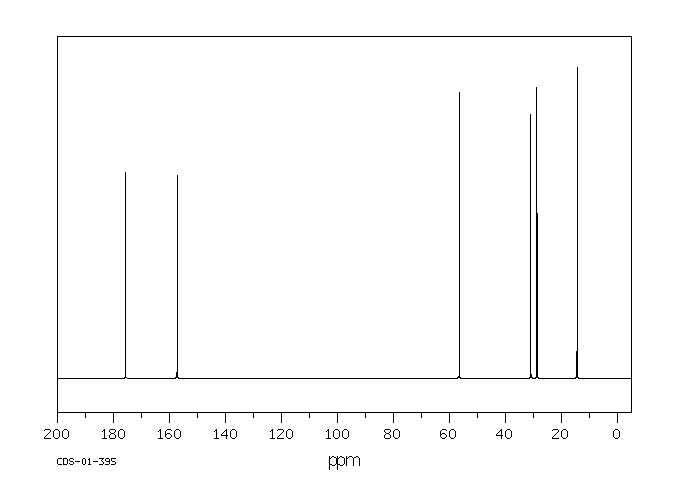
碳谱13CNMR
-
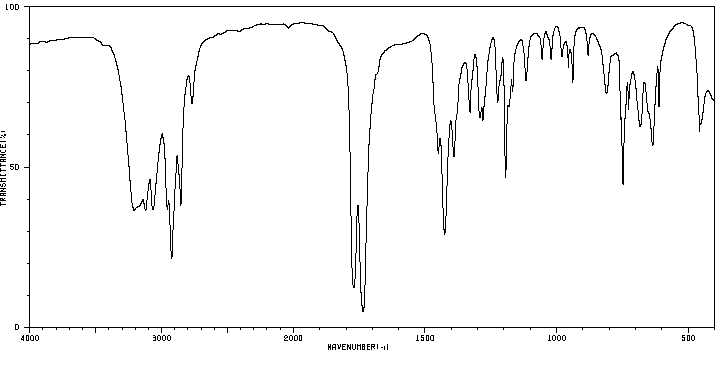
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-4-异丙基-2-恶唑烷硫酮
麻黄恶碱
顺-八氢-2H-苯并咪唑-2-酮
顺-1-(4-氟苯基)-4-[1-(4-氟苯基)-4-羰基-1,3,8-三氮杂螺[4.5]癸-8-基]环己甲腈
非达司他
降冰片烯缩醛3-((1S,2S,4S)-双环[2.2.1]庚-5-烯-2-羰基)恶唑烷-2-酮
阿齐利特
阿那昔酮
阿洛双酮
阿帕鲁胺
阿帕他胺杂质2
铟烷-2-YL-甲基胺盐酸
钾3-{2-[3-氰基-3-(十二烷基磺酰基)-2-丙烯-1-亚基]-1,3-噻唑烷-3-基}-1-丙烷磺酸酯
钠2-{[4,5-二羟基-3-(羟基甲基)-2-氧代-1-咪唑烷基]甲氧基}乙烷磺酸酯
重氮烷基脲
詹氏催化剂
解草恶唑
解草噁唑
表告依春
螺莫司汀
螺立林
螺海因氮丙啶
螺[咪唑烷-4,3'-吲哚啉]-2,2',5-三酮
螺[1-氮杂双环[2.2.2]辛烷-8,5'-咪唑烷]-2',4'-二酮
苯甲酸,4-氟-,2-[5,7-二(三氟甲基)-1,8-二氮杂萘-2-基]-2-甲基酰肼
苯氰二硫酸,1-氰基-1-甲基-4-氧代-4-(2-硫代-3-噻唑烷基)丁酯
苯妥英钠杂质8
苯妥英钠
苯妥英-D10
苯妥英
苯基硫代海因半胱氨酸钠盐
苯基硫代乙内酰脲-谷氨酸
苯基硫代乙内酰脲-蛋氨酸
苯基硫代乙内酰脲-苯丙氨酸
苯基硫代乙内酰脲-色氨酸
苯基硫代乙内酰脲-脯氨酸
苯基硫代乙内酰脲-缬氨酸
苯基硫代乙内酰脲-异亮氨酸
苯基硫代乙内酰脲-天冬氨酸
苯基硫代乙内酰脲-亮氨酸
苯基硫代乙内酰脲-丙氨酸
苯基硫代乙内酰脲-D-苏氨酸
苯基硫代乙内酰脲-(NΕ-苯基硫代氨基甲酰)-赖氨酸
苯基乙内酰脲-甘氨酸
苏氨酸-1-(苯基硫基)-2,4-咪唑烷二酮(1:1)
色氨酸标准品002
膦酸,(2-羰基-1-咪唑烷基)-,二(1-甲基乙基)酯
脱氢-1,3-二甲基尿囊素
脱氢-1,3,8-三甲基尿囊素
聚(d(A-T)铯)