1-氟金刚烷 | 768-92-3
中文名称
1-氟金刚烷
中文别名
——
英文名称
1-adamantyl fluoride
英文别名
1-fluoroadamantane;adamantyl fluoride
CAS
768-92-3
化学式
C10H15F
mdl
MFCD02682106
分子量
154.228
InChiKey
CPWSNJSGSXXVLD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:225 °C
-
沸点:188.8±9.0 °C(Predicted)
-
密度:1.06±0.1 g/cm3(Predicted)
-
保留指数:1159;1159;1174;1184;1196;1159;1159
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:11
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2903890090
-
危险品标志:Xi
-
储存条件:室温且干燥
SDS
1-Fluoroadamantane Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1-Fluoroadamantane
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1-Fluoroadamantane
Percent: >98.0%(GC)
CAS Number: 768-92-3
Synonyms: 1-Adamantyl Fluoride
Chemical Formula: C10H15F
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
1-Fluoroadamantane
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:257°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
1-Fluoroadamantane
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen fluoride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
1-Fluoroadamantane
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1-Fluoroadamantane
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1-Fluoroadamantane
Percent: >98.0%(GC)
CAS Number: 768-92-3
Synonyms: 1-Adamantyl Fluoride
Chemical Formula: C10H15F
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
1-Fluoroadamantane
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:257°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
1-Fluoroadamantane
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen fluoride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
1-Fluoroadamantane
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法:
- 制法:
- 聚氟化氢吡啶(2): 将37.5g (0.475mol) 吡啶加入到一个干燥的250mL聚乙烯瓶中,用干冰-丙酮浴冷却至吡啶固化。然后缓慢加入87.5g (4.37mol) 无水氟化氢,旋转反应瓶以促使固体溶解。待完全溶解后,可安全地慢慢升温至室温。从而得到聚氟化氢吡啶(2)。
- 1-氟金刚烷(1): 在一个250mL的聚乙烯反应瓶中加入5g (0.033mol) 1-金刚烷醇和50mL上述聚氟化氢吡啶(2)溶液,室温搅拌3小时。随后加入150mL石油醚并剧烈搅拌15分钟。使用聚乙烯分液漏斗分离下层无机相,并用大量水洗涤。接着用水、饱和碳酸氢钠溶液及水依次洗涤有机物后,再用无水硫酸镁干燥。经减压浓缩后,可获得约4.5~4.6g (1-氟金刚烷) 1,收率约为88%~90%。该产物可通过升华或甲醇-四氯化碳重结晶进行提纯。
- 注: 使用此方法可以将仲醇、叔醇转化为氟代烃,收率可达70%~90%。聚氟化氢-吡啶试剂还可直接使烯烃、环丙烷、重氮化合物等发生氢氟化加成反应,生成有机氟化物。
合成制备方法:
- 制法:
- 聚氟化氢吡啶(2): 将37.5g (0.475mol) 吡啶加入干燥的250mL聚乙烯瓶中,在干冰-丙酮浴冷却至固化后,缓慢添加87.5g (4.37mol) 无水氟化氢。通过旋转反应瓶促使固体溶解,随后安全升温至室温。得到聚氟化氢吡啶(2)。
- 1-氟金刚烷(1): 在一个250mL的聚乙烯反应瓶中加入5g (0.033mol) 1-金刚烷醇和50mL上述聚氟化氢吡啶(2)溶液,室温下搅拌3小时。接着加入150mL石油醚并剧烈搅拌15分钟,利用聚乙烯分液漏斗分离下层无机相,并用大量水破坏。有机物依次用水、饱和碳酸氢钠溶液及水洗涤后,再进行无水硫酸镁干燥处理。减压浓缩后,可获得约4.5~4.6g (1-氟金刚烷) 1,收率约为88%~90%,并可用升华或甲醇-四氯化碳重结晶的方法提纯。
- 注: 此方法可用于将仲醇、叔醇转化为氟代烃,收率可达70%~90%。聚氟化氢-吡啶试剂同样能直接使烯烃、环丙烷和重氮化合物发生氢氟化加成反应,生成有机氟化物。
用途简介: 暂无内容。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-氟-2-金刚烷酮 5-fluoroadamantan-2-one 41171-83-9 C10H13FO 168.211 —— 1-fluoro-3-chloro-adamantane 60389-52-8 C10H14ClF 188.673 —— 1-Fluoro-3-bromoadamantane 60389-54-0 C10H14BrF 233.124 —— 3-fluoroadamantan-1-ol 58652-35-0 C10H15FO 170.227 —— 1-Fluoro-2-adamantanone 41171-84-0 C10H13FO 168.211
反应信息
-
作为反应物:参考文献:名称:亲电性苯氧基取代的phospho阳离子†摘要:电苯氧基取代的磷鎓盐的家庭[(RO)P(C 6 ˚F 5)3 ] [B(C 6 ˚F 5)4 ](R = C 6 H ^ 5,4-FC 6 H ^ 4,2,4- -F 2 C 6 H 3,C 6 F 5)已被合成并评估了它们的空气稳定性。已使用氟离子亲和力和整体亲电指数的计算来比较这些phospho盐的亲电性。这些磷鎓盐的路易斯酸度在弗里德-克来福特型二聚,加氢脱氟,加氢甲硅烷基化,加氢脱氧和脱氢偶联反应中进行了计算和实验研究。DOI:10.1039/c6dt03544b
-
作为产物:描述:1-金刚烷甲酸 在 4-二甲氨基吡啶 、 Ir(dFppy)3 、 triethylamine tris(hydrogen fluoride) 、 N,N'-二异丙基碳二亚胺 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 生成 1-氟金刚烷参考文献:名称:通过光氧化还原催化实现的自由基-极性交叉对氧化还原活性酯进行亲核(放射性)氟化摘要:我们报告了在可见光照射下使用 Ir 光催化剂对 N-羟基邻苯二甲酰亚胺酯进行亲核氟化的氧化还原中性方法。该方法提供了获得广泛的脂肪族氟化物的途径,包括伯、仲和叔苄基氟化物以及未活化的叔氟化物,由于竞争消除,这些氟化物通常无法通过亲核氟化获得。此外,我们表明脱羧氟化条件很容易适应 [18F]KF 的放射性氟化。我们建议反应通过 Ir 催化剂和氧化还原活性酯底物之间的两次电子转移进行,以提供碳阳离子中间体,随后被氟化物捕获。DOI:10.1021/jacs.0c03125
-
作为试剂:参考文献:名称:Subcomponent Exchange Transforms an FeII4L4 Cage from High- to Low-Spin, Switching Guest Release in a Two-Cage System摘要:Subcomponent exchange transformed new high-spin (Fe4L4)-L-II cage 1 into previously-reported low-spin (Fe4L4)-L-II cage 2: 2-formyl-6-methylpyridine was ejected in favor of the less sterically hindered 2-formylpyridine, with concomitant high- to low-spin transition of the cage's Fell centers. High-spin 1 also reacted more readily with electron-rich anilines than 2, enabling the design of a system consisting of two cages that could release their guests in response to combinations of different stimuli. The addition of p-anisidine to a mixture of high-spin 1 and previously-reported low-spin (Fe4L4)-L-II cage 3 resulted in the destruction of 1 and the release of its guest. However, initial addition of 2-formylpyridine to an identical mixture of 1 and 3 resulted in the transformation of 1 into 2; added p-anisidine then reacted preferentially with 3 releasing its guest. The addition of 2-formylpyridine thus modulated the system's behavior, fundamentally altering its response to the subsequent signal p-anisidine.DOI:10.1021/jacs.7b01478
文献信息
-
A Series of Deoxyfluorination Reagents Featuring OCF<sub>2</sub> Functional Groups作者:Shiyu Zhao、Yong Guo、Zhaoben Su、Wei Cao、Chengying Wu、Qing-Yun ChenDOI:10.1021/acs.orglett.0c03238日期:2020.11.6utilization of decomposition products of PFECAs. We report herein a new series of deoxyfluorination reagents featuring OCF2 functional groups derived from certain PFECAs. Alkyl fluorides were generated from various alcohols in ≤97% yield by these novel reagents. The mechanistic experiment verified in situ generation of carbonic difluoride (COF2).
-
Widely Applicable Hydrofluorination of Alkenes via Bifunctional Activation of Hydrogen Fluoride作者:Zhichao Lu、Xiaojun Zeng、Gerald B. Hammond、Bo XuDOI:10.1021/jacs.7b12704日期:2017.12.20Expanding the use of fluorine in pharmaceuticals, agrochemicals and materials requires a widely applicable and more efficient protocol for the preparation of fluorinated compounds. We have developed a new generation nucleophilic fluorination reagent, KHSO4-13HF, HF 68 wt/wt %, that is not only easily handled and inexpensive but also capable of hydrofluorinating diverse, highly functionalized alkenes
-
Photoredox-catalyzed deoxyfluorination of activated alcohols with Selectfluor®作者:María González-Esguevillas、Javier Miró、Jenna L. Jeffrey、David W.C. MacMillanDOI:10.1016/j.tet.2019.05.043日期:2019.8Herein we disclose a deoxyfluorination of alcohols with an electrophilic fluorine source via visible-light photoredox catalysis. This radical-mediated C–F coupling is capable of fluorinating secondary and tertiary alcohols efficiently, complementing previously reported nucleophilic deoxyfluorination protocols.
-
Hindered dialkyl ether synthesis with electrogenerated carbocations作者:Jinbao Xiang、Ming Shang、Yu Kawamata、Helena Lundberg、Solomon H. Reisberg、Miao Chen、Pavel Mykhailiuk、Gregory Beutner、Michael R. Collins、Alyn Davies、Matthew Del Bel、Gary M. Gallego、Jillian E. Spangler、Jeremy Starr、Shouliang Yang、Donna G. Blackmond、Phil S. BaranDOI:10.1038/s41586-019-1539-y日期:2019.9.19simple route towards the synthesis of hindered ethers, in which electrochemical oxidation is used to liberate high-energy carbocations from simple carboxylic acids. These reactive carbocation intermediates, which are generated with low electrochemical potentials, capture an alcohol donor under non-acidic conditions; this enables the formation of a range of ethers (more than 80 have been prepared here)受阻醚在各种应用中都具有很高的价值;然而,它们仍然是未充分探索的化学空间领域,因为它们难以通过常规反应合成 1,2。这种基序在药物化学中非常令人垂涎,因为对醚键的广泛取代可以防止可能导致体内快速降解的不需要的代谢过程。在这里,我们报告了合成受阻醚的简单途径,其中电化学氧化用于从简单的羧酸中释放高能碳正离子。这些反应性碳阳离子中间体以低电化学势生成,在非酸性条件下捕获醇供体;这使得能够形成一系列醚(这里已经制备了 80 多种),否则很难获得。碳正离子也可以被简单的亲核试剂拦截,导致受阻醇甚至烷基氟化物的形成。评估该方法能够规避制备 12 种化学支架时遇到的合成瓶颈,从而提高所需产品的产率,此外还显着减少了制备所需的步骤数量和劳动量。分子探针的使用和动力学研究的结果支持了所提出的机制和添加剂在所检查条件下的作用。我们在这里报告的反应流形证明了电化学在温和条件下获得高反应性中间体的能力,反过来,
-
Borane-Catalyzed C(sp<sup>3</sup>)–F Bond Arylation and Esterification Enabled by Transborylation作者:Dominic R. Willcox、Gary S. Nichol、Stephen P. ThomasDOI:10.1021/acscatal.1c00282日期:2021.3.19given the high thermodynamic barrier to C–F bond cleavage. Stoichiometric hydridoborane-mediated C–F functionalization has recently emerged, but is yet to be rendered catalytic. Herein, the borane-catalyzed coupling of alkyl fluorides with arenes (carbon–carbon bond formation) and carboxylic acids (carbon–oxygen bond formation) has been developed using transborylation reactions to achieve catalytic鉴于氟碳键断裂的高热力学障碍,碳氟键的活化和功能化是一个重大的合成挑战。化学计量氢化硼硼烷介导的CF功能化最近出现,但尚未被催化。在本文中,已经开发出了硼烷催化的烷基氟与芳烃(形成碳-碳键)和羧酸(形成碳-氧键)的偶联反应,以实现催化转化。在各种结构上和电子分化芳烃和羧酸的使用9-硼杂双环[3.3.1]壬烷(H-达到成功的C-C和C-O耦合乙-9-BBN)作为催化剂和频哪醇硼烷(HBpin),具有宽泛的官能团耐受性。实验和计算研究表明碳-碳和碳-氧偶联反应的机理二分法。乙-F transborylation(B-F / B-H复分解)F-之间乙-9-BBN和HBpin启用碳-碳键形成的催化周转,而烷基氟化物和acyloxyboronic酯之间的直接交换(C-F / B -O复分解)被提议用于碳-氧偶联,其中H - B -9-BBN催化羧酸与HBpin的脱氢偶联。
表征谱图
-
氢谱1HNMR
-
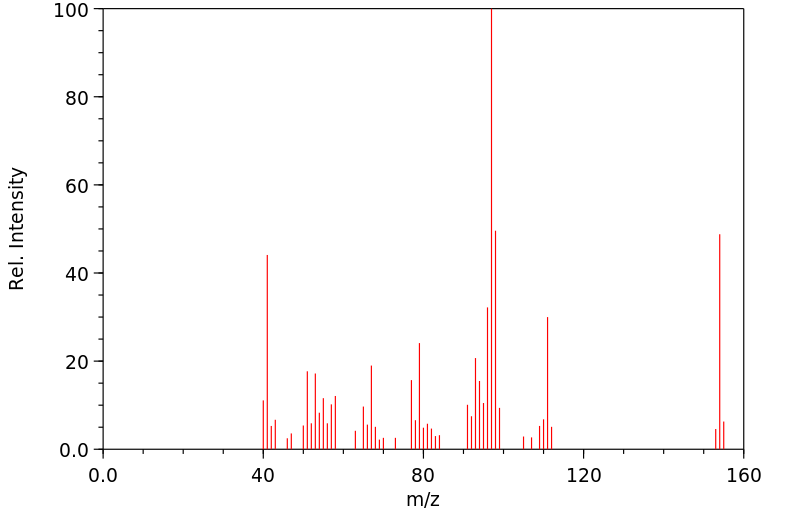
质谱MS
-
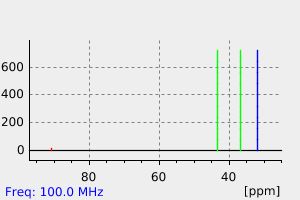
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-氟-环丙胺
顺式-1,1,1,4,4,4-六氟-2-丁烯
顺-1,1,2,2,3,4-六氟环丁烷
酰亚胺基二亚磷酸,甲基-,四(2,2,2-三氟乙基)酯
舒巴坦酸
聚(7-脱氮杂腺嘌呤酸)
癸烷,6-溴-1,1,1,2,2,3,3-七氟-4,4-二(三氟甲基)-
环丙基溴化镁
溴五氟乙烷
氯氟烃-252
氯氟烃-232
氯氟-甲基
氯四氟乙烷
氯二氟乙醛
氯三氟乙烷
氨甲酸,(氟磺酰)-,甲基酯
氢氯氟碳-261
氟甲醇
氟甲基自由基
氟甲基环戊烷
氟甲基环丙烷
氟环辛烷
氟环戊烷
氟环庚烷
氟环十二烷
氟环丁烷
1-溴-1-氯-2,2,2-三氟乙烷
氟氯乙烷
氟化烯丙基
氟化乙亚胺酰基,2-(二氟氨基)-N,2,2-三氟-
氟化丁基
氟乙醛
氟乙烷
氟乙烯醚
正膦胺,N-(2,3,4,5,6-五氯-2,3,4,5,6-五氟亚环己基)-1,1,1,1-四(2,2,3,3-四氟丙氧基)-
桉叶素
替氟烷
恩氟烷
异氟醚
异十八烷酸己酯
己酸,2,5-二氨基-6-羟基-(7CI)
奥替尼啶HCL
壬氟环戊烷
地氟烷
叔丁基氟化物
反式-2-氟环丙胺盐酸盐
反式-2-氟代环戊烷-1-胺盐酸盐
反式-1,2-双(全氟己基)乙烯
反式-1,2-双(全氟-n-丁基)乙烯
反式-1,1,1,2,2,3,3-七氟-4-壬烯