3-(4-甲基苯基)-1,2-恶唑-5(4H)-酮 | 25755-82-2
中文名称
3-(4-甲基苯基)-1,2-恶唑-5(4H)-酮
中文别名
——
英文名称
3-(p-tolyl)isoxazol-5(4H)-one
英文别名
3-p-Tolyl-isoxazol-5-on;3-(4-Methylphenyl)-5(4H)-isoxazolone;3-(4-methylphenyl)-4H-1,2-oxazol-5-one
CAS
25755-82-2
化学式
C10H9NO2
mdl
——
分子量
175.187
InChiKey
IADHZJJQULYQGB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:38.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-苯基-5-异噁唑酮 3-phenyl-4H-isoxazol-5-one 1076-59-1 C9H7NO2 161.16
反应信息
-
作为反应物:描述:参考文献:名称:1-(2H-Azirine-2-羰基)苯并三唑:合成含吡咯杂环的基石摘要:开发了一种一锅法制备2 H - azirine-2-羰基苯并三唑,该方法是通过苯并三唑与2 H -azirine-2-羰基氯的反应形成的,该反应是由Fe(II)催化的苯甲酰异构化生成的。 5-氯异恶唑。2 H-叠氮基-2-羰基苯并三唑与1,3-二酮的Co(II)催化反应以中等到良好的产率提供了2-(((苯并三唑-1-基)羰基)吡咯。碱促进的2-(((苯并三唑-1-基)羰基)吡咯与醛,酮,异氰酸酯和异硫氰酸酯的碱环合反应提供各种取代的吡咯并[1,2- c ]恶唑和1 H-吡咯并[1,2- c]咪唑衍生物,中等至高收率。这些加合物的6-酰基可被三氟甲磺酸除去,得到进一步的新的吡咯并稠合的O-和N-杂环,如6-未取代的吡咯并[1,2 - c ]恶唑-1(3 H)-和1 H-吡咯并[1,2- c ]咪唑-1,3(2 H)-二酮,而1 H-吡咯并[1,2- c ]咪唑-1,3(2 H当用POCl 3DOI:10.1039/d0ob00206b
-
作为产物:描述:参考文献:名称:异恶唑酮与异氰酸酯的钯催化扩环反应:1,3-氧杂嗪-6-一衍生物的合成摘要:公开了异恶唑酮与异氰酸酯的钯催化的扩环反应。在反应中,提出了涉及开环/环化的级联过程。该反应由于没有消除CO 2而具有高原子经济性。此外,所获得的产品展示出具有较高固态发射效率的聚集诱发的发射特性。DOI:10.1002/adsc.202001200
文献信息
-
Rh(III)-Catalyzed Regioselective Annulations of 3-Arylisoxazolones and 3-Aryl-1,4,2-dioxazol-5-ones with Propargyl Alcohols: Access to 4-Arylisoquinolines and 4-Arylisoquinolones作者:Tong-Tong Wang、Hai-Shan Jin、Man-Man Cao、Ru-Bing Wang、Li-Ming ZhaoDOI:10.1021/acs.orglett.1c02049日期:2021.8.6The Rh(III)-catalyzed dual directing group assisted C–H activation/annulation of 3-arylisoxazolones with propargyl alcohols has been developed, which expands the application scope of isoxazolones in organic synthesis. This protocol also worked well with 3-aryl-1,4,2-dioxazol-5-ones to produce synthetically and biologically important 4-arylisoquinolones.
-
Reformatsky and Blaise reactions in flow as a tool for drug discovery. One pot diversity oriented synthesis of valuable intermediates and heterocycles作者:L. Huck、M. Berton、A. de la Hoz、A. Díaz-Ortiz、J. AlcázarDOI:10.1039/c6gc02619b日期:——The application of Reformatsky and Blaise reactions for the preparation of a diverse set of valuable intermediates and heterocycles in a one-pot protocol is described. To achieve this goal, a...描述了Reformatsky和Blaise反应在单罐实验方案中用于制备各种有价值的中间体和杂环的应用。为了实现这个目标,...
-
Rh(III)-Catalyzed [4 + 2] Annulation of 3-Aryl-5-isoxazolone with Maleimides or Maleic Ester作者:Ting Wan、Chao Pi、Yangjie Wu、Xiuling CuiDOI:10.1021/acs.orglett.0c02283日期:2020.8.21The Rh(III)-catalyzed [4 + 2] annulation of 3-aryl-5-isoxazolones with maleimides or maleic ester has been developed, which gives synthetically important 3,4-dihydroisoquinoline derivatives in good to excellent yields. This facile protocol can tolerate a variety of functional groups, and CO2 was produced as the predominant byproduct. Notably, a C–C bond and a C–N bond were formed simultaneously. This
-
Atom-Economic Silver-Catalyzed Difunctionalization of the Isocyano Group with Cyclic Oximes: Towards Pyrimidinediones作者:Hong-Wen Liang、Zhen Yang、Kun Jiang、Ying Ye、Ye WeiDOI:10.1002/anie.201801363日期:2018.5.14An unprecedented silver‐catalyzed difunctionalization of the isocyano group with cyclic oximes is described. This method allows efficient and atom‐economic assembly of a vast array of structurally novel and interesting pyrimidinediones, and tolerates a range of functionalities. The resulting products can be easily converted into some useful compounds. Furthermore, the method can also be applied for
-
Construction of isoxazolone-fused phenanthridines <i>via</i> Rh-catalyzed cascade C–H activation/cyclization of 3-arylisoxazolones with cyclic 2-diazo-1,3-diketones作者:Wangcheng Hu、Xinwei He、Tongtong Zhou、Youpeng Zuo、Shiwen Zhang、Tingting Yang、Yongjia ShangDOI:10.1039/d0ob02310h日期:——A Rh(III)-catalyzed cascade C–H activation/intramolecular cyclization of 3-aryl-5-isoxazolones with cyclic 2-diazo-1,3-diketones was described, leading to the formation of isoxazolo[2,3-f]phenanthridine skeletons. The protocol features the simultaneous one-pot formation of two new C–C/C–N bonds and one heterocycle in moderate-to-good yields with good functional group compatibility. It is amenable to
表征谱图
-
氢谱1HNMR
-
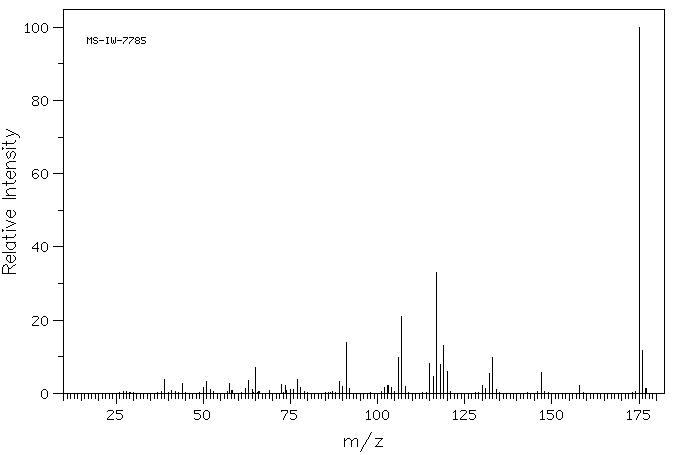
质谱MS
-
碳谱13CNMR
-
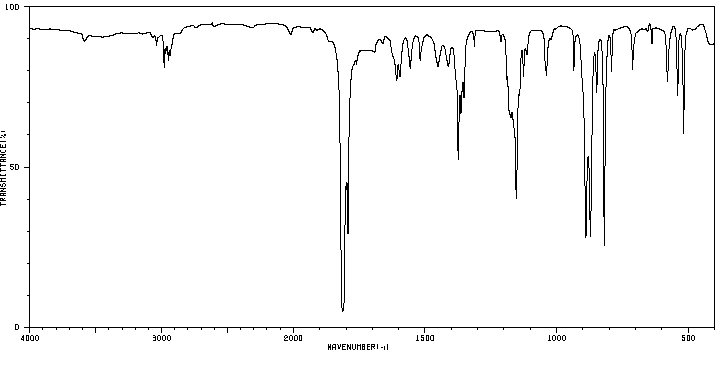
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫