Sodium DL-malate
中文名称
——
中文别名
——
英文名称
Sodium DL-malate
英文别名
disodium;2-hydroxybutanedioate
CAS
——
化学式
C4H4Na2O5
mdl
——
分子量
178.05
InChiKey
WPUMTJGUQUYPIV-UHFFFAOYSA-L
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-9.75
-
重原子数:11
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:101
-
氢给体数:1
-
氢受体数:5
反应信息
-
作为反应物:描述:Sodium DL-malate 以 水 为溶剂, 以7.8 μL of 4M malic acid aqueous solution obtained的产率得到苹果酸参考文献:名称:Method of immobilizing malate dehydrogenase on a substrate摘要:将苹果酸脱氢酶固定在底物上,使用含有苹果酸脱氢酶的甘油溶液通过将混合溶液滴入甘油中,混合溶液中至少添加苹果酸和苹果酸盐中的一种,然后在底物上干燥。最好是通过将苹果酸盐添加到含有苹果酸脱氢酶的甘油溶液中来制备混合溶液。苹果酸盐最好是选择钾苹果酸盐和钠苹果酸盐中的至少一种。公开号:US07521214B2
-
作为产物:描述:参考文献:名称:摘要:DOI:
文献信息
-
Method of introducing amino group and method of synthesizing amino acid compound申请人:——公开号:US20040162434A1公开(公告)日:2004-08-19The present invention relates to a method for introducing an amino group into an organic acid or an organic ester by reacting an organic salt or an organic ester and ammonia under high-temperature and high-pressure water conditions, a method for synthesizing an amino acid or an amino ester by the above method, and a method for manufacturing an amino acid compound by synthesizing an amino acid or an amino ester by the above method and separating and refining it with an ion exchange resin.
-
Crystalline forms of lamotrigine申请人:Hanna Mazen公开号:US08486927B2公开(公告)日:2013-07-16The invention is directed to novel crystalline forms of lamotrigine. These novel crystalline forms of lamotrigine have improved dissolution and in-vivo absorption profile, as compared to pure lamotrigine. These novel crystalline forms of lamotrigine provide a substantial increase in the blood concentration of lamotrigine, as compared to pure lamotrigine when administered to a subject. These novel crystalline forms of lamotrigine also provide a slower, steady build up of lamotrigine blood concentration suitable for sustained release of lamotrigine in-vivo, as compared to pure lamotrigine.
-
Crystal of 5-hydroxy-1H-imidazole-4-carboxamide 3/4 hydrate, method for producing the same and crystal of 5-hydroxy-1H-imidazole-4-carboxamide hydrate申请人:FUJIFILM Corporation公开号:US09108928B2公开(公告)日:2015-08-18Provided is a method for producing 5-hydroxy-1H-imidazole-4-carboxamide.3/4 hydrate, including: reacting 2-aminomalonamide with a compound represented by the following formula [1] in the presence of a carboxylic acid to obtain 5-hydroxy-1H-imidazole-4-carboxamide: wherein, in formula [1], each R independently represents a C1-3 alkyl group; reacting the resulting 5-hydroxy-1H-imidazole-4-carboxamide with an acidic compound to obtain a 5-hydroxy-1H-imidazole-4-carboxamide acid salt or a hydrate thereof; and reacting the resulting 5-hydroxy-1H-imidazole-4-carboxamide acid salt or hydrate thereof with a salt in the presence of an acidic solvent to obtain the 5-hydroxy-1H-imidazole-4-carboxamide.3/4 hydrate.
-
CRYSTAL OF 5-HYDROXY-1H-IMIDAZOLE-4-CARBOXAMIDE 3/4 HYDRATE, METHOD FOR PRODUCING THE SAME AND CRYSTAL OF 5-HYDROXY-1H-IMIDAZOLE-4-CARBOXAMIDE HYDRATE申请人:FUJIFILM CORPORATION公开号:US20150315156A1公开(公告)日:2015-11-05Provided is a method for producing 5-hydroxy-1H-imidazole-4-carboxamide.3/4 hydrate, including: reacting 2-aminomalonamide with a compound represented by the following formula [1] in the presence of a carboxylic acid to obtain 5-hydroxy-1H-imidazole-4-carboxamide: wherein, in formula [1], each R independently represents a C 1-3 alkyl group; reacting the resulting 5-hydroxy-1H-imidazole-4-carboxamide with an acidic compound to obtain a 5-hydroxy-1H-imidazole-4-carboxamide acid salt or a hydrate thereof; and reacting the resulting 5-hydroxy-1H-imidazole-4-carboxamide acid salt or hydrate thereof with a salt in the presence of an acidic solvent to obtain the 5-hydroxy-1H-imidazole-4-carboxamide.3/4 hydrate.
-
Crystalline Forms of lamotrigine申请人:Hanna Mazen公开号:US20090176787A1公开(公告)日:2009-07-09The invention is directed to novel crystalline forms of lamotrigine. These novel crystalline forms of lamotrigine have improved dissolution and in-vivo absorption profile, as compared to pure lamotrigine. These novel crystalline forms of lamotrigine provide a substantial increase in the blood concentration of lamotrigine, as compared to pure lamotrigine when administered to a subject. These novel crystalline forms of lamotrigine also provide a slower, steady build up of lamotrigine blood concentration suitable for sustained release of lamotrigine in-vivo, as compared to pure lamotrigine.
表征谱图
-
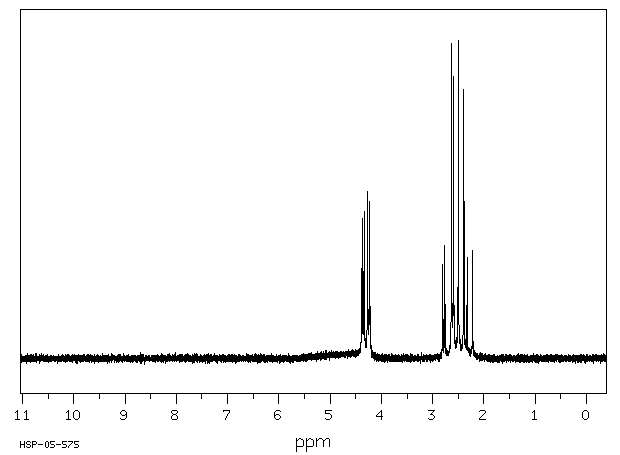
氢谱1HNMR
-
质谱MS
-
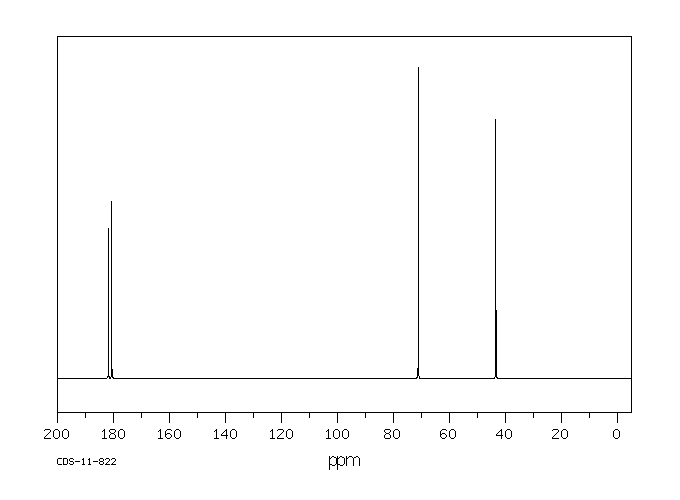
碳谱13CNMR
-
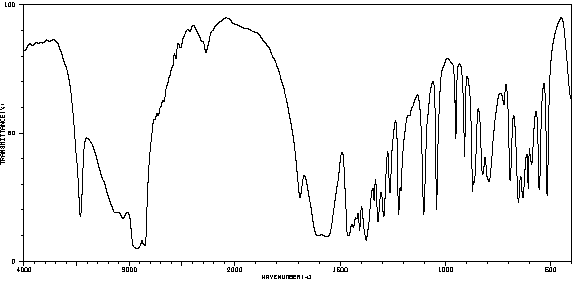
红外IR
-
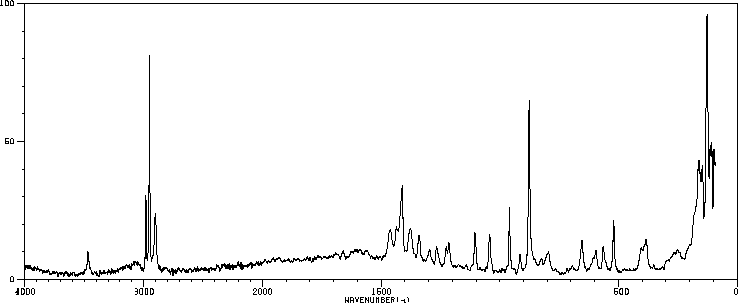
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2-苯基-3-羟基丙酸
(2S,3R)-2,3-二羟基-3-(2-吡啶基)丙酸乙酯,N-氧化物
麦拉乳酸
阿拉伯碳酸氢二钾
铵;铈(+3)阳离子;(2R,3R)-2,3-二羟基丁烷二酸盐
钡二{8-[3-(2-羟基辛基)-2-环氧乙烷基]辛酸酯}
钠3-脱氧-D-阿拉伯糖-己酮酸酯
钠3-脱氧-D-木糖基-己酮酸酯
钠(3R,5R)-3,5-二羟基-7-[(1S,2S,6R,8S,8aR)-8-羟基-2,6-二甲基-1,2,6,7,8,8A-六氢-1-萘基]庚酸酯
钠(2S)-2-羟基(13C3)丙酸酯
酮酯
酒石酸锂单水合物
酒石酸铬
酒石酸铜(II)一水
酒石酸钾锑
酒石酸钾
酒石酸钠
酒石酸鐵(III)鉀
酒石酸辛酯钠盐
酒石酸羟吡啶
酒石酸氢钾
酒石酸异丙酯
酒石酸二磺基琥珀酰亚胺酯
酒石酸二琥珀酰亚胺酯
酒石酸二戊酯
酒石酸二仲丁酯
酒石酸二丙酯
辛酸,8-氯-6-羟基-,(6R)-
辛伐他汀钾盐
辛伐他汀钠盐
辛伐他汀酸
超支化BIS-MPA聚酯-64-羟基,4代
西托溴铵
表洛伐他汀羟基酸钠盐
葡萄糖酸镍
葡萄糖酸锶
葡萄糖酸锰
葡萄糖酸汞
葡萄糖酸亚铁
莫那可林J酸
苹果酸镁
苹果酸镁
苹果酸铵盐
苹果酸钙
苹果酸氢钠
苹果酸氢钠
苹果酸根
苹果酸二烯丙酯
苹果酸二乙基己酯
苹果酸乙酯(S)-2-羟基丁二酸1-乙酯(苹果酸杂质S)