Calcium 2-hydroxy-4-(methylthio)butano
中文名称
——
中文别名
——
英文名称
Calcium 2-hydroxy-4-(methylthio)butano
英文别名
calcium;2-hydroxy-4-methylsulfanylbutanoate
CAS
——
化学式
C10H18CaO6S2
mdl
——
分子量
338.5
InChiKey
ABRVDWASZFDIEH-UHFFFAOYSA-L
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-2.68
-
重原子数:19
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:171
-
氢给体数:2
-
氢受体数:8
文献信息
-
2-METHYLTHIOETHYL-SUBSTITUTED HETEROCYCLES AS FEED ADDITIVES申请人:Kobler Christoph公开号:US20090162474A1公开(公告)日:2009-06-25A chemical compound of the general formula I or II is useful as feed additive: wherein X=O or NR, and R=H, C 1 -C 6 -alkyl (optionally branched), C 3 -C 6 -cycloalkyl, aryl, or aralkyl, and wherein R 1 , R 2 , are identical or different and in each case H, C 1 -C 6 -alkyl (optionally branched), C 3 -C 6 -cycloalkyl, allyl, aryl, or aralkyl; or R 1 and R 2 together are an C 2 - to C 6 -alkylene group (optionally C 1 -C 6 -alkyl substituted).通用公式 I 或 II 的化合物作为饲料添加剂是有用的:其中 X=O 或 NR,R=H、C1-C6-烷基(可选支链)、C3-C6-环烷基、芳基或芳基烷基,R1、R2 相同或不同,在每种情况下为 H、C1-C6-烷基(可选支链)、C3-C6-环烷基、烯丙基、芳基或芳基烷基;或者 R1 和 R2 一起是 C2 到 C6 烷基烯基(可选 C1-C6 烷基取代)。
-
Process for optically active 3-(methane-sulfonyloxy) thiolane and analogs申请人:Pfizer Inc.公开号:US04954647A1公开(公告)日:1990-09-04Intermediates and a stepwise process for the conversion of D-methionine or certain of its derivatives to optically active compounds of the formula ##STR1## wherein R is (C.sub.1 -C.sub.3)alkyl, phenyl or tolyl. The latter compounds are in turn useful as an intermediate in the preparation of penem antibiotic 5R,6S-6-(1R-hydroxyethyl)-2-(1R-oxo-3S-thiolanylthio)-2-penem-3-carboxylic acid and corresponding pharmaceutically acceptable salts and esters.
-
Nonionic X-ray contrast agents, compositions and methods申请人:Mallinckrodt, Inc.公开号:US05075502A1公开(公告)日:1991-12-24Triiodinated isophthalamide derivatives, useful as X-ray contrast agents, having at least one amide group derived from the amino-alcohol, 3-(N-2-hydroxyethyl)amino-1,2-propanediol, which provides high water solubility and low mammalian toxicity, and methods of preparing them. Methods of preparing 3-(N-2-hydroxyethyl)amino-1,2-propanediol are provided.
-
Process for optically active 3-(methane-sulfonyloxy)thiolane and analogs申请人:Pfizer Inc.公开号:US04874877A1公开(公告)日:1989-10-17Intermediates and a stepwise process for the conversion of D-methionine or certain of its derivatives to optically active compounds of the formula ##STR1## wherein R is (C.sub.1 -C.sub.3) alkyl, phenyl or tolyl. The latter compounds are in turn useful as an intermediate in the preparation of penem antibiotic 5R,6S-6-(1R-hydroxyethyl)-2-(1R-oxo-3S-thiolanylthio)-2-penem-3-carboxylic acid and corresponding pharmaceutically acceptable salts and esters.
-
Inhibitors of protein isoprenyl transferases申请人:University of Pittsburgh公开号:US06310095B1公开(公告)日:2001-10-30Compounds having the formula or a pharmaceutically acceptable salt thereof wherein R1 is (a) hydrogen, (b) loweralkyl, (c) alkenyl, (d) alkoxy, (e) thioalkoxy, (f) halo, (g) haloalkyl, (h) aryl-L2—, and (i) heterocyclic-L2—; R2 is selected from (a) (b) —C(O)NH—CH(R14)—C(O)OR15, (d) —C(O)NH—CH(R14)—C(O)NHSO2R16, (e) —C(O)NH—CH(R14)-tetrazolyl, (f) —C(O)NH-heterocyclic, and (g) —C(O)NH—CH(R14)—C(O)NR17R18; R3 is substituted or unsubstituted heterocyclic or aryl, substituted or unsubstituted cycloalkyl or cycloalkenyl, and —P(W)RR3RR3′; R4 is hydrogen, lower alkyl, haloalkyl, halogen, aryl, arylakyl, heterocyclic, or (heterocyclic)alkyl; L1 is absent or is selected from (a) —L4—N(R5)—L5—, (b) —L4—O—L5—, (c) —L4—S(O)n—L5— (d) —L4—L6—C(W)—N(R5)—L5—, (e) —L4—L6—S(O)m—N(R5)—L5 —, (f) —L4—N(R5)—C(W)—L7—L5—, (g) —L4—N(R5)—S(O)p—L7—L5—, (h) optionally substituted alkylene, (i) optionally substituted alkenylene, (j) optionally substituted alkynylene (k) a covalent bond, (l) and (m) are inhibitors of protein isoprenyl transferases. Also disclosed are protein isoprenyl transferase inhibiting compositions and a method of inhibiting protein isoprenyl transferases.具有以下化学式或其药学上可接受的盐,其中R1为(a)氢,(b)低碳基,(c)烯基,(d)烷氧基,(e)硫代烷氧基,(f)卤素,(g)卤代烷基,(h)芳基-L2-,以及(i)杂环-L2-;R2选自(a)、(b)、—C(O)NH—CH(R14)—C(O)OR15、(d)、—C(O)NH—CH(R14)—C(O)NHSO2R16、(e)、—C(O)NH—CH(R14)-四唑基、(f)、—C(O)NH-杂环,以及(g)、—C(O)NH—CH(R14)—C(O)NR17R18;R3是取代或未取代的杂环或芳基,取代或未取代的环烷基或环烯基,以及—P(W)RR3RR3′;R4为氢、低碳基、卤代烷基、卤素、芳基、芳基烷基、杂环或(杂环)烷基;L1不存在或选自(a)、—L4—N(R5)—L5—、(b)、—L4—O—L5—、(c)、—L4—S(O)n—L5—、(d)、—L4—L6—C(W)—N(R5)—L5—、(e)、—L4—L6—S(O)m—N(R5)—L5—、(f)、—L4—N(R5)—C(W)—L7—L5—、(g)、—L4—N(R5)—S(O)p—L7—L5—、(h)、可选取代的烷基、(i)、可选取代的烯基、(j)、可选取代的炔基、(k)、共价键、(l)和(m)是蛋白异戊烯基转移酶的抑制剂。还公开了蛋白异戊烯基转移酶抑制剂组合物和一种抑制蛋白异戊烯基转移酶的方法。
表征谱图
-
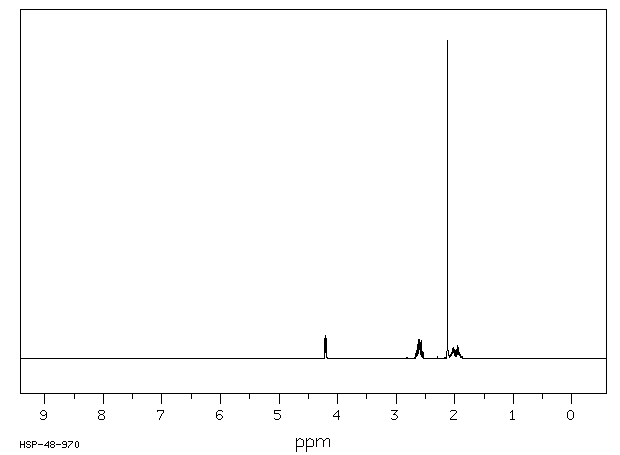
氢谱1HNMR
-
质谱MS
-
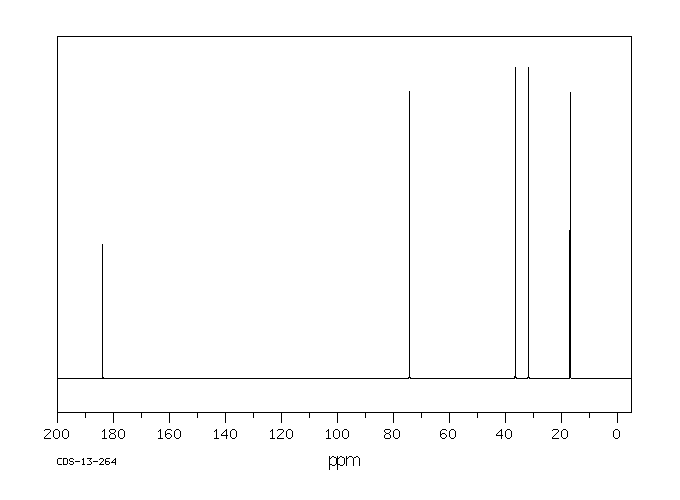
碳谱13CNMR
-
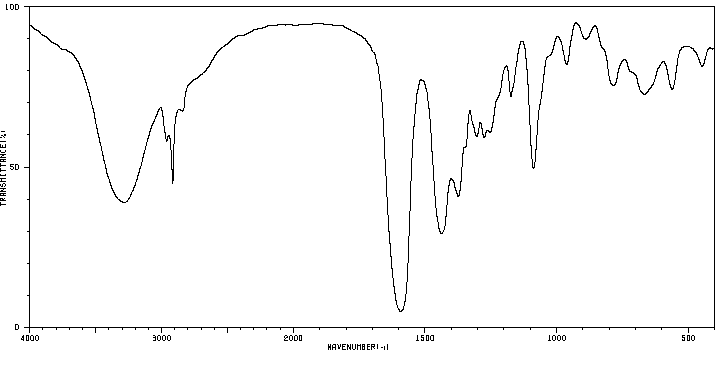
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯