p-甲氧基苄烯-p-氰基苯胺 | 13036-19-6
中文名称
p-甲氧基苄烯-p-氰基苯胺
中文别名
4-[(4-甲氧基苯亚甲基)氨基]氰苯;4-[(4-甲氧亚苄基)氨基]苄腈
英文名称
p-methoxybenzylidene-p-cyano-aniline
英文别名
4-[(4-methoxybenzylidene)amino]cyanobenzene;p-(methoxybenzylidene)-p-n-cyanoaniline;p-methoxybenzylidene p'-cyanoaniline;4-(4-methoxy-benzylidenamino)-benzonitrile;4-(4-Methoxy-benzylidenamino)-benzonitril;4-[(4-methoxy-benzylidene)-amino]-benzonitrile;4-[(4-Methoxybenzylidene)amino]benzonitrile;4-[(4-methoxyphenyl)methylideneamino]benzonitrile
CAS
13036-19-6
化学式
C15H12N2O
mdl
——
分子量
236.273
InChiKey
SZTAFPBCQLNZQR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:106 °C
-
沸点:419.6±30.0 °C(Predicted)
-
密度:1.05±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:45.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2926909090
-
储存条件:室温且干燥环境下使用。
SDS
4-[(4-Methoxybenzylidene)amino]benzonitrile Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 4-[(4-Methoxybenzylidene)amino]benzonitrile
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Harmful if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Do not eat, drink or smoke when using this product.
[Prevention]
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 4-[(4-Methoxybenzylidene)amino]benzonitrile
>98.0%(T)
Percent:
CAS Number: 13036-19-6
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Synonyms: 4-(p-Anisalamino)benzonitrile , 4-[(p-Anisylidene)amino]benzonitrile , N-(p-
Anisylidene)-4-cyanoaniline , 4-(4-Methoxybenzylidene)-4-cyanoaniline
Chemical Formula: C15H12N2O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal- Powder
Colour: Very pale yellow - Pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 4-[(4-Methoxybenzylidene)amino]benzonitrile
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Harmful if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Do not eat, drink or smoke when using this product.
[Prevention]
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 4-[(4-Methoxybenzylidene)amino]benzonitrile
>98.0%(T)
Percent:
CAS Number: 13036-19-6
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Synonyms: 4-(p-Anisalamino)benzonitrile , 4-[(p-Anisylidene)amino]benzonitrile , N-(p-
Anisylidene)-4-cyanoaniline , 4-(4-Methoxybenzylidene)-4-cyanoaniline
Chemical Formula: C15H12N2O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal- Powder
Colour: Very pale yellow - Pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
席夫碱[液晶(LC)材料]
反应信息
-
作为反应物:描述:参考文献:名称:一种亚胺加氢还原制备仲胺类化合物的方法摘要:本发明属于医药和天然化合物化工中间体及相关化学技术领域,提供了一种亚胺加氢还原制备仲胺类化合物的方法。本发明以亚胺及其衍生物为原料、纳米多孔钯为催化剂、氢气为氢源,高效地加氢还原亚胺化合物,其中氢气的压力为0.1~20.0MPa;N‑苄烯基苯亚胺及其衍生物在溶剂中的摩尔浓度为0.01~2mmol/mL。所采用的催化剂孔骨架大小为1‑50nm之间,亚胺及其衍生物与所用催化剂摩尔比为1:0.01~1:0.5。本发明的有益效果是该产物产率高,反应条件非常温和,操作和后处理简单,催化剂重复性好,且多次使用催化效果没有明显降低,为其实现工业化提供可能。公开号:CN110483308B
-
作为产物:描述:参考文献:名称:取代基的交互作用对取代的亚苄基苯胺中C N桥基的电子特性的影响-介晶化合物分子核的模型。甲13 C NMR研究和比较与理论结果摘要:在CDCl 3中,对一系列介晶分子模型化合物(即C 13)测量了13 C NMR化学位移δC (C N)。取代的亚苄基苯胺p -X C 6 H 4 CH NC 6 H 4 p -Y(X = NO 2,CN,CF 3,F,Cl,H,Me,MeO或NMe 2; Y = NO 2,CN ,F,Cl,H,Me,MeO或NMe 2)。δ的取代基依赖性Ç(CN)被用作研究电子取代基对偶氮甲碱单元的作用的工具。亚苄基取代基X有δ的反向效应Ç(C N):吸电子原因屏蔽的取代基,而给电子性的人的行为相反,感应效果清楚地在共振效应为主。相反,苯胺取代基Y发挥正常作用:吸电子取代基引起屏蔽,而供电子取代基引起C N碳屏蔽,感应效应和共振效应的强度非常相似。此外,可以验证X和Y之间是否存在特定的交叉相互作用。相邻芳环取代基的电子效应可系统地改变C的灵敏度N基团对亚苄基或苯胺环取代基的电子作用。吸电子苯胺环上的取代基降低δ的灵敏度Ç(CDOI:10.1021/jo0600508
文献信息
-
[DE] SÄUREGRUPPEN-SUBSTITUIERTE DIPHENYLAZETIDINONE, VERFAHREN ZU DEREN HERSTELLUNG, DIESE VERBINDUNGEN ENTHALTENDE ARZNEIMITTEL UND DEREN VERWENDUNG<br/>[EN] DIPHENYL AZETIDINONES SUBSTITUTED BY ACIDIC GROUPS, METHOD FOR THEIR PRODUCTION, MEDICAMENTS CONTAINING SAID COMPOUNDS AND USE THEREOF<br/>[FR] DIPHENYLAZETINIDONES SUBSTITUEES PAR DES GROUPES ACIDES, PROCEDE DE FABRICATION, MEDICAMENTS CONTENANT CES COMPOSES ET UTILISATION DE CES MEDICAMENTS申请人:AVENTIS PHARMA GMBH公开号:WO2004000805A1公开(公告)日:2003-12-31Die Erfindung betrifft Verbindungen der Formel (I), worin R1, R2, R3, R4, R5 , and R6 die angegebenen Bedeutungen haben, sowie deren physiologisch verträgliche Salze. Die Verbindungen eignen sich z.B. als Hypolipidämika.该发明涉及式(I)的化合物,其中R1、R2、R3、R4、R5和R6具有所指的含义,以及它们的生理耐受盐。这些化合物适合用作降血脂药。
-
[DE] KATIONISCH SUBSTITUIERTE DIPHENYLAZETIDINONE, VERFAHREN ZU DEREN HERSTELLUNG, DIESE VERBINDUNGEN ENTHALTENDE ARZNEIMITTEL UND DEREN VERWENDUNG<br/>[EN] CATIONICALLY SUBSTITUTED DIPHENYL AZETIDINONES, METHOD FOR THEIR PRODUCTION, MEDICAMENTS CONTAINING SAID COMPOUNDS AND USE THEREOF<br/>[FR] DIPHENYLACETINIDONES A SUBSTITUTION CATIONIQUE, PROCEDE DE FABRICATION, MEDICAMENTS CONTENANT CES COMPOSES ET UTILISATION申请人:AVENTIS PHARMA GMBH公开号:WO2004000803A1公开(公告)日:2003-12-31Die Erfindung betrifft Verbindungen der Formel I, (I) worin R1, R2, R3, R4, R5 , und R6 die angegebenen Bedeutungen haben, sowie deren physiologisch verträgliche Salze. Die Verbindungen eignen sich z.B. als Hypolipidämika.该发明涉及公式I的化合物,其中R1,R2,R3,R4,R5和R6具有所述的含义,以及其生理相容的盐。这些化合物可用作降血脂药物。
-
[DE] RINGSUBSTITUIERTE DIPHENYLAZETIDINONE, VERFAHREN ZU DEREN HERSTELLUNG, DIESE VERBINDUNGEN ENTHALTENDE ARZNEIMITTEL UND DEREN VERWENDUNG<br/>[EN] RING-SUBSTITUTED DIPHENYL AZETIDINONES, METHOD FOR THE PRODUCTION THEREOF, MEDICAMENTS CONTAINING SAID COMPOUNDS, AND USE THEREOF<br/>[FR] DIPHENYLAZETIDINONES A NOYAU SUBSTITUE, PROCEDES POUR LES PRODUIRE, MEDICAMENTS CONTENANT CES COMPOSES ET LEUR UTILISATION申请人:AVENTIS PHARMA GMBH公开号:WO2004000804A1公开(公告)日:2003-12-31Ringsubstituierte Diphenylazetidinone der Formel I, Verfahren zu deren Herstellung, diese Verbindungen enthaltende Arzneimittel und deren Verwendung zur Behandlung von Hyperlipidämie sowie Arteriosklerose und Hypercholesterinämie.
-
Nano-tube TiO2 as a new catalyst for eco-friendly synthesis of imines in sunlight作者:Mona Hosseini-SarvariDOI:10.1016/j.cclet.2010.11.017日期:2011.5Nanomaterials are considered as suitable heterogeneous catalysts for many organic reactions. Herein nano-tube TiO2 has been reported as a heterogeneous catalyst, for synthesis of imines in sunlight at room temperature under solvent-free conditions. The condensation of less electrophilic carbonyl compounds with poorly nucleophilic amines was afforded imines in excellent yields. (c) 2010 Mona Hosseini-Sarvari. Published by Elsevier B.V. on behalf of Chinese Chemical Society. All rights reserved.
-
Synthesis of Optically Pure <i>vic</i>-Sulfanyl Amines Mediated by a Remote Sulfinyl Group作者:Yolanda Arroyo、M. Ascensión Sanz-Tejedor、Inés Alonso、José L. García-RuanoDOI:10.1021/ol201696y日期:2011.9.2Enantiomerically pure syn-1,2-diaryl-1,2-sulfanylamine derivatives can be obtained in a completely stereoselective manner by reaction of the benzylcarbanion Li-(S)-1 with N-phenyl (or PMP)-arylidene aldimines and further desulfinylation with t-BuLi. Theoretical studies at the DFT (mPW1PW91) level with the CPCM model, by using the Gaussian09 program, provide a good explanation for the stereochemical results.
表征谱图
-
氢谱1HNMR
-
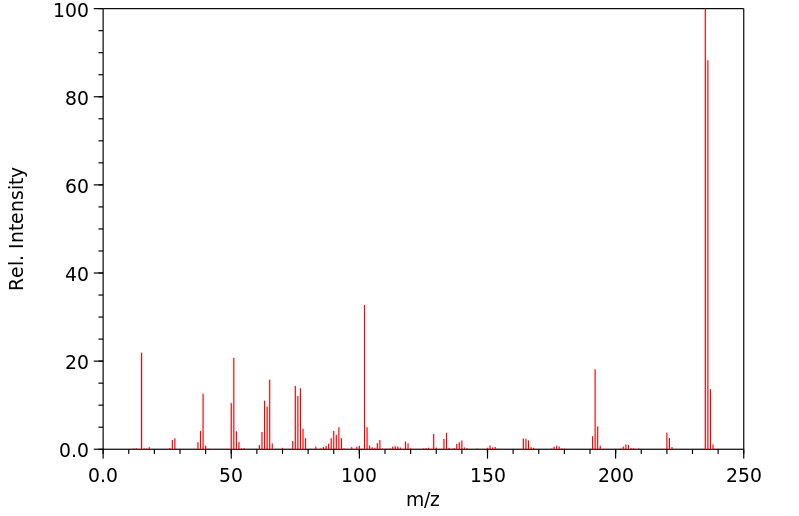
质谱MS
-
碳谱13CNMR
-
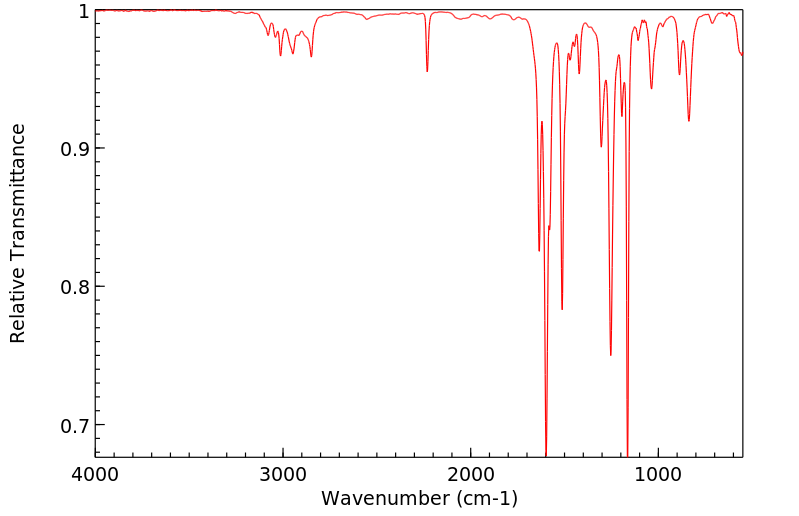
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫