一水合肼 | 10217-52-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-2.01
-
重原子数:3
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:53
-
氢给体数:3
-
氢受体数:3
ADMET
制备方法与用途
-
尿素法:将次氯酸钠与氢氧化钠按一定比例混合配成溶液,边搅拌边加入尿素与少量高锰酸钾的混合液,直接通蒸汽加热至103~104℃进行氧化反应。反应液中含肼量20g/L,经蒸馏、真空浓缩制得40%水合肼,再经烧碱脱水、减压蒸馏,制得80%水合肼。
-
氨法:以氨水及次氯酸钠为原料。氨水中加入0.1%骨胶,抑制肼的过度分解。将次氯酸钠按氨与次氯酸钠摩尔比值为(50~60)∶1迅速加入氨水中,在强烈搅拌下于常压或高压下进行氧化反应,先生成氯胺,后继续反应生成肼。反应液经蒸馏回收氨,再经真空蒸馏除去氯化钠及氢氧化钠,蒸发气体冷凝为低浓度肼,再分馏制得不同浓度水合肼。
-
注意事项:水合肼的热蒸汽与空气接触会引起爆炸,因此包括蒸馏水合肼在内的实验应在氮气流中进行。其蒸气对眼睛、皮肤和呼吸道有刺激作用,应于通风橱内操作。将100g硫酸水合肼和等质量的KOH粉末放入蒸馏烧瓶中,加入5mL水,在通氮气的情况下加热反应至完全。所得水合肼含有水分,可通过分馏除去。
- 尿素法:将次氯酸钠与氢氧化钠按一定比例混合配制成溶液,边搅拌边加入尿素及少量高锰酸钾的混合液,直接通蒸汽加热至103~104℃进行氧化反应。反应液中含肼量20g/L,经蒸馏、真空浓缩制得40%水合肼,再经过烧碱脱水和减压蒸馏制得80%水合肼。
反应信息
-
作为反应物:描述:参考文献:名称:NH 3,N 2 H 4,HNO 3和CH 3 NH 2的ArF激光光解中光碎屑发射的激发机理的确定摘要:在泵浦探针实验中,使用两个受激准分子激光器研究了由标题分子产生电子激发的光片段的机理。我们从NH 3,N 2 H 4和CH 3 NH 2中找到NH(A);来自HNO 3和CN(B)的OH(A)和来自CH 3 NH 2的CH(A,B)涉及初级光片段的二次激发。DOI:10.1016/0009-2614(85)80187-1
-
作为产物:参考文献:名称:通过调节单原子铁位点的电子分布来提高氮到氨的电还原动力学摘要:电催化氮还原反应(NRR)在下一代电化学能量转换技术中起着至关重要的作用。但是,NRR动力学仍然受到无贵金属电催化剂加氢反应缓慢的限制。在此,我们报告了一种合理的设计和合成方法,该方法的混合催化剂的原子铁位点固定在具有反蛋白石结构的N,O掺杂的多孔碳(Fe SA ‐NO-C)基质上,从而可显着提高NH 3的收率。 31.9微克的速率ħ -1 毫克-1猫。NRR电催化在-0.4 V时的法拉第效率为11.8%,几乎超过了以前报道的所有原子分散的金属-氮-碳催化剂。理论计算表明,Fe SA ‐ NO-C催化剂具有较高的NRR催化活性,这主要是由于均匀分布在多孔碳载体上的相邻O和Fe原子之间的电荷转移优化,这不仅显着促进了运输N 2和离子的结合,但也有效降低了分离的Fe原子与* N 2中间体之间的结合能以及速率确定步骤(* N 2 →* NNH)的热力学吉布斯自由能。DOI:10.1002/anie.202100526
-
作为试剂:参考文献:名称:Effective Synthesis of N-Alkyl-3-(Indol-3-yl)Pyrazoles from Ag2CO3-Catalyzed Regioselective Aza-Michael Addition of 5-(Indol-3-yl)-1HPyrazoles摘要:
Abstract: In this study, the synthesis of N-alkyl-3-(indol-3-yl)pyrazoles was carried out from Ag2CO3 catalyzed regioselective aza-Michael addition of 5-(indol-3-yl)-1H-pyrazoles to a, bunsaturated carbonyl compounds. In the presence of 10 mol% of Ag2CO3, the reaction smoothly occurred in dichloroethane (DCE) at 120oC to preferentially afford a series of N-alkyl-3-(indol-3- yl)pyrazoles in high yields with good regioselectivity. It was found that 1-methyl-3-(3-methyl-1Hpyrazol- 5-yl)-2-phenyl-1H-indole, 1-benzyl-3-(3-methyl-1H-pyrazol-5-yl)-1H-indole, a, b-unsaturated ketone, and a, b-unsaturated amide exclusively gave 3-(pyrazol-3-yl)indoles in good yields. This reaction features high regioselectivity, mild reaction conditions, good substrate scope and yields, and a commercially available catalyst. Meanwhile, the reaction was also proven to be quite practical by the gram-scale synthesis of N-alkyl-3-(indol-3-yl)pyrazoles in excellent yields with good regioselectivity.
DOI:10.2174/0115701786295074240305095904
文献信息
-
Synthesis of ferrocene amides and esters from aminoferrocene and 2-substituted ferrocenecarboxylic acid and properties thereof作者:Palabindela Srinivas、Sunchu Prabhakar、Floris Chevallier、Ekhlass Nassar、William Erb、Vincent Dorcet、Viatcheslav Jouikov、Palakodety Radha Krishna、Florence MonginDOI:10.1039/c6nj02018f日期:——aminoferrocene and various acid coupling partners such as Nα-Boc-L-tryptophan and N-protected sugar amino acids (N-Boc-3-amino-3-deoxy-1,2-O-isopropylidene-α-D-ribofuranoic acid, N-Boc-3-amino-3-deoxy-1,2-O-isopropylidene-α-D-xylofuranoic acid and their corresponding homo- and hetero-dimers). Similarly, reactions between 2-aminoethyl ferrocenecarboxylate and N-protected sugar amino acids afforded compounds不同ferrocenecarboxamides从aminoferrocene和各种酸偶联伴侣比如合成ñ α -Boc-大号色氨酸和ñ -保护的糖的氨基酸(Ñ -Boc-3-氨基-3-脱氧-1,2- ö异亚丙基α - d -ribofuranoic酸,ñ -Boc-3-氨基-3-脱氧-1,2- ö异亚丙基α- d -xylofuranoic酸和它们相应的均聚物和杂二聚体)。类似地,2-氨基乙基二茂铁羧酸酯和N之间的反应-被保护的糖氨基酸提供了具有远离二茂铁核心定位的羧酰胺官能团的化合物。其中一个的X射线衍射结构表明在酰胺官能团之间存在分子间氢键。制备羰基氨基(或羰氧基)和氧羰基1,2-二取代的二茂铁,以外消旋混合物形式或对映体纯(S P)形式。他们的电化学评估显示出鲜明的特征。有趣的是,在存在L-谷氨酸的情况下,对映体纯的二茂铁二酯显示出大的电位偏移(+ 45mV)。最后,评估了一些合成的二
-
Aryl substituted pyrazoles, and pyrroles, and the use thereof申请人:Euro-Celtique S.A.公开号:US06414011B1公开(公告)日:2002-07-02This invention relates to compounds having the Formula I: or a pharmaceutically acceptable salt, prodrug or solvate thereof, wherein Het and R5-R13 are set in the specification. The invention also is directed to the use of compounds of Formula I for the treatment of neuronal damage following global and focal ischemia, for the treatment or prevention of neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS), and for the treatment, prevention or amelioration of both acute or chronic pain, as antitinnitus agents, as anticonvulsants, and as antimanic depressants, as local anesthetics, as antiarrhythmics and for the treatment or prevention of diabetic neuropathy.
-
Main Chain Ferrocenyl Amides from 1‐Aminoferrocene‐1′‐carboxylic Acid作者:Katja Heinze、Manuela SchlenkerDOI:10.1002/ejic.200300897日期:2004.7The non-natural amino acid 1-aminoferrocene-1′-carboxylic acid is synthesised from ferrocene in eight steps. The folding and association phenomena of amido-substituted ferrocenes in the crystal as well as in solution are studied by X-ray crystallography, IR and NMR spectroscopy and DFT calculations. The amino acid is selectively protected at the amino group with the use of the fluorenyl-9-methoxycarbonyl
-
Reduction of Steroid Ketones and other Carbonyl Compounds by Modified Wolff--Kishner Method作者:[not available]. Huang-MinlonDOI:10.1021/ja01178a008日期:1949.10concentration of the regenerated ketone a t a low level.2 In fact all the C3-steroid ketones so far investigated gave the normal C3-methylenic compounds by the modified Wolff-Kish20-diketopregnane (I) gave a reduced product melting a t 152-153' in which one of the two keto groups is unattacked. In view of the fact that the 20-keto compounds, such as A5-pregnen-3 (P)-ol20-one (IV) and its hydrogenation product根据Dutcher 和Wintersteiner2,在通常条件下通过Wow-Kishner 方法还原C3-类固醇酮主要产生相应的Cs-差向异构甲醇和少量预期的Cs-亚甲基产物。在α,P-不饱和酮、胆甾酮的情况下,还原遵循更复杂的过程。除了差向异构不饱和甲醇和少量正常产物A4-胆甾醇外,还分离出饱和甲醇、α-粪甾醇和P-胆甾醇。作者指出,异常还原可以通过以下假设来解释:腙或缩氨基脲部分水解为游离酮,然后被醇钠还原为仲醇。然而,这些可能性被修改后的 WolffKishner red ~ ction 消除,~ , ~ 其中水在加热期间蒸发。此外,该反应中使用过量的水合肼应将再生酮的浓度保持在较低水平。 2 事实上,迄今为止研究的所有 C3-甾体酮都通过改性的 Wolff-Kish20-二酮孕烷得到了正常的 C3-亚甲基化合物(I) 得到在 152-153' 熔化的还原产物,其中两个酮基中的一个未
-
[EN] N-ACYLHYDRAZONE DERIVATIVES FOR SELECTIVE T CELL INHIBITOR AND ANTI-LYMPHOID MALIGNANCY DRUG<br/>[FR] DÉRIVÉS DE N-ACYLHYDRAZONE DESTINÉS À UN MÉDICAMENT ANTIMALIGNITÉ LYMPHOÏDE ET INHIBANT SÉLECTIVEMENT LES LYMPHOCYTES T申请人:CHONG KUN DANG PHARM CORP公开号:WO2014035149A1公开(公告)日:2014-03-06The present invention relates to novel N-acylhydrazone derivatives, and more particularly to novel N-acylhydrazone derivatives having selective T cell inhibitory activity and/or anti-lymphoid malignancy activity, stereoisomers thereof, pharmaceutically acceptable salts thereof, the use thereof for preparing pharmaceutical compositions, pharmaceutical compositions containing the same, treatment methods using the compositions, and methods for preparing the novel N-acylhydrazone derivatives.
表征谱图
-
氢谱1HNMR
-
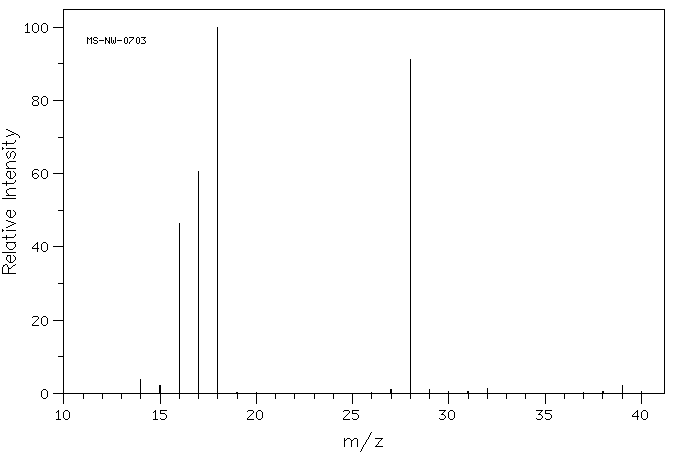
质谱MS
-
碳谱13CNMR
-
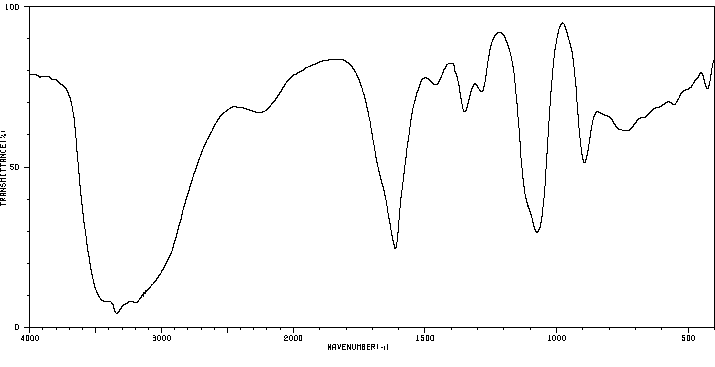
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息