三正丁基碘化锍 | 18146-62-8
中文名称
三正丁基碘化锍
中文别名
三丁基碘化锍;三正丁基锍碘
英文名称
tributylsulfonium iodide
英文别名
Tributyl-sulfonium; Jodid;tributylsulfanium;iodide
CAS
18146-62-8
化学式
C12H27S*I
mdl
——
分子量
330.317
InChiKey
HUCQSHDLMUWBPS-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:93 °C
-
溶解度:在甲醇中几乎透明
计算性质
-
辛醇/水分配系数(LogP):1.01
-
重原子数:14
-
可旋转键数:9
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2930909090
SDS
三丁基碘化锍 修改号码:5
模块 1. 化学品
产品名称: Tributylsulfonium Iodide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 三丁基碘化锍
百分比: >96.0%(T)
CAS编码: 18146-62-8
分子式: C12H27IS
三丁基碘化锍 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
光敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 极淡的黄色-浅黄色
三丁基碘化锍 修改号码:5
模块 9. 理化特性
气味: 无资料
pH: 无数据资料
熔点:
93°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 碘化氢, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
三丁基碘化锍 修改号码:5
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Tributylsulfonium Iodide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 三丁基碘化锍
百分比: >96.0%(T)
CAS编码: 18146-62-8
分子式: C12H27IS
三丁基碘化锍 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
光敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 极淡的黄色-浅黄色
三丁基碘化锍 修改号码:5
模块 9. 理化特性
气味: 无资料
pH: 无数据资料
熔点:
93°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 碘化氢, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
三丁基碘化锍 修改号码:5
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:参考文献:名称:Expanded Halogen-Bonded Anion Organic Networks with Star-Shaped Iodoethynyl-Substituted Molecules: From Corrugated 2D Hexagonal Lattices to Pyrite-Type 2-Fold Interpenetrated Cubic Lattices摘要:Halogen bonding interactions between halide anions and neutral polyiodinated linkers are used for the elaboration of anion organic frameworks, by analogy with well-known MOF derivatives. The extended, 3-fold symmetry, 1,3,5-tris(iodoethynyl)-2,4,6-trifluorobenzene (1) cocrystallizes with a variety of halide salts, namely, Et3S+I-, Et3MeN+I-, Et4N+Br-, Et3BuN+Br-, Me-DABCO(+)I(-), Bu3S+I-, Bu4N+Br-, Ph3S+Br-, Ph4P+Br-, and PPN+Br-. Salts with 1:1 stoichiometry formulated as (1).(C+,X-) show recurrent formation of corrugated (6,3) networks, with the large cavities thus generated, filled either by the cations and solvent (CHCl3) molecules and/or by interpenetration (up to 4-fold interpenetration). The 2:1 salt formulated as (1)(2)center dot(Et3BuN+Br-) crystallizes in the cubic Ia (3) over bar space group (a = 22.573(5) angstrom, V = 11502(4) angstrom(3)), with the Br- ion located on (3) over bar site and molecule 1 on a 3-fold axis. The 6-fold, unprecedented octahedral coordination of the bromide anion generates an hexagonal three-dimensional network of Pa (3) over bar symmetry, as observed in the pyrite model structure, at variance with the usual, but lower-symmetry, rutile-type topology. In this complex system, the I centering gives rise to a 2-fold interpenetration of class Ia, while the cations and solvent molecules are found disordered within interconnected cavities. Another related cubic structure of comparable unit cell volume (space group Pa (3) over bar, a = 22.4310(15) angstrom, V = 11286.2(13) angstrom(3)) is found with (1)(2)center dot(Et3S+I-).DOI:10.1021/ja400740v
-
作为产物:参考文献:名称:Molten and Solid Trialkylsulfonium Iodides and Their Polyiodides as Electrolytes in Dye-Sensitized Nanocrystalline Solar Cells摘要:Potential new electrolytes for dye-sensitized nanocrystalline solar cells (DNSCs) of Gratzel type based on trialkylsulfonium iodides have been investigated. Room temperature molten salts of (Et2MeS)I, (Bu2MeS)I, and (Bu2EtS)I, with iodine in low concentrations, revealed good conducting abilities. DNSCs using iodine-doped (Bu2MeS)l as electrolyte achieved an overall light-to-electricity conversion efficiency of 3.7% in simulated AM 1.5 solar light at a light intensity of 0.1 Sun. The effects from varying the temperature during the IN measurements were studied, as well as the effects of 4-tert-butylpyridine treatment of the electrodes.DOI:10.1021/jp036859v
-
作为试剂:描述:参考文献:名称:Sulfonium Salts as Liquid-Liquid Phase-Transfer Catalysts摘要:月基二甲基磺鎓盐和三(正丁基)磺鎓盐作为高效催化剂,用于两相反应。DOI:10.1055/s-1988-27594
文献信息
-
Living radical polymerization initiator, method for producing polymer, and polymer申请人:KYOTO UNIVERSITY公开号:US10414848B2公开(公告)日:2019-09-17A living radical polymerization initiator represented by the following: wherein, R1 represents an aromatic, an alkylcarbonyl, an alkoxycarbonyl, an aminocarbonyl, an alkylaminocarbonyl, a dialkylaminocarbonyl, an arylcarbonyl, an alkylsulfonyl, an aryl sulfonyl or combinations thereof; R2, R3, R4 and R5 are a hydrogen, an aliphatic, an aromatic, an alkylcarbonyl, an alkoxycarbonyl, an aminocarbonyl, an alkylaminocarbonyl, a dialkylaminocarbonyl, an arylcarbonyl, a carboxy, an alkylsulfonyl or an aryl sulfonyl; X and Y are a halogen; m and n are an integer of 1 or more, and are non-symmetrical so that X and Y have different reactivities to initiate a living radical polymerization.
-
Room Temperature Molten Salts Based on Trialkylsulfonium Cations and Bis(trifluoromethylsulfonyl)imide作者:Hajime Matsumoto、Toshihiko Matsuda、Yoshinori MiyazakiDOI:10.1246/cl.2000.1430日期:2000.12Symmetric and aliphatic trialkylsulfonium cation form room temperature molten salts (RTMS) with bis(trifluoromethylsulfonyl)imide (TFSI). In particular RTMS based on triethylsulfonium shows low melting point (−35 °C) and the lowest viscosity (30 mPs at 25 °C) of all the TFSI based RTMS as reported so far. The conductivity of 7.1 mS cm−1 at 25 °C is the highest of all the non-chloroaluminate RTMS based on aliphatic onium cations.
-
Polymerizable composition, optical material comprising the composition and method for producing the material申请人:——公开号:US20040254258A1公开(公告)日:2004-12-16A cured matter obtained by polymerizing and curing a polymerizable composition comprising a compound having at least one episulfide in a molecule is reduced in an odor by adding a perfume to the polymerizable composition comprising the compound having at least one episulfide in a molecule. A cloudiness of the lens is removed by raising a purity of sulfur to 98% or more. A reduction in a chlorine content of the sulfur compound described above to 0.1% by weight or less elevates an oxidation resistance and a light fastness of the high refractive index optical material and improves a color tone thereof. An optical product having a high refractive index and a high-degree transparency is provided by subjecting the composition in advance to deaerating treatment at 0 to 100° C. for one minute to 24 hours under a reduced pressure of 0.001 to 50 torr.
-
A process for preparing epoxy resins from aliphatic hydroxyl-containing compounds申请人:THE DOW CHEMICAL COMPANY公开号:EP0121260A2公开(公告)日:1984-10-10The present invention is a process for preparing an epoxy resin which comprises reacting (A) a material having an average of more than one aliphatic hydroxyl group per molecule with (B) an epoxy alkyl halide in the presence of (C) an alkali metal hydroxide and (D) at least one phase transfer catalyst, removing the salt formed, allowing the reaction to separate into an aqueous phase and an organic phase, removing the aqueous phase, and recovering the epoxy resin from the organic phase. The invention is characterized by repeating at least once the steps in which the organic phase is reacted with a quantity of component (C) sufficient to cause a separation of the reaction mixture into an aqueous phase and an organic phase and which is at least stoichiometric with the amount of unreacted component (A), removing the saltformed, allowing the reaction mixture to separate into an aqueous phase and an organic phase, and removing the aqueous phase. This process provides for a high level of conversion of the aliphatic hydroxyl-containing material to the corresponding glycidyl ethers.
-
Novel heptamethyl indane compound
表征谱图
-
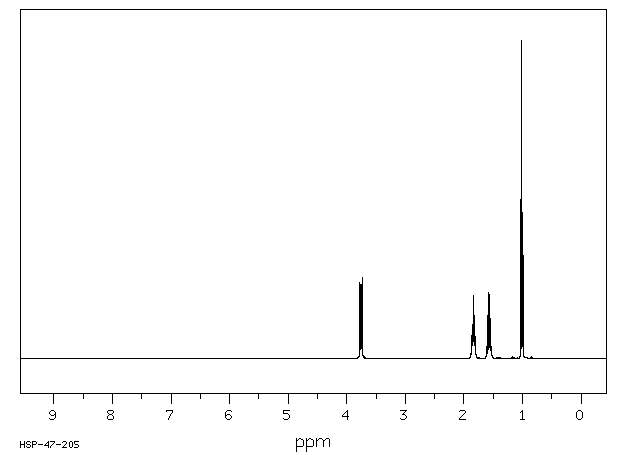
氢谱1HNMR
-
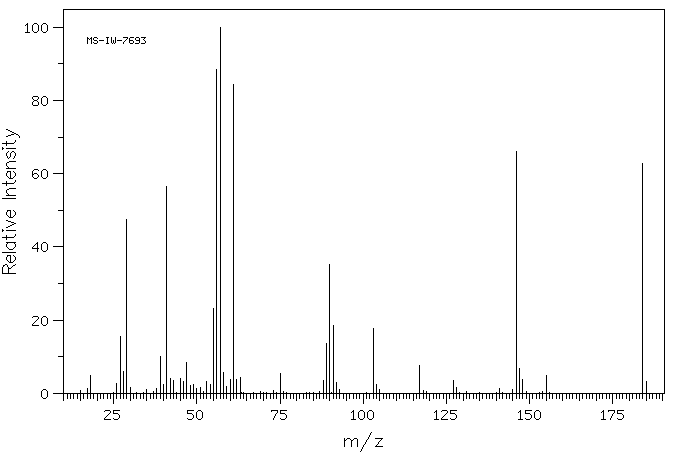
质谱MS
-
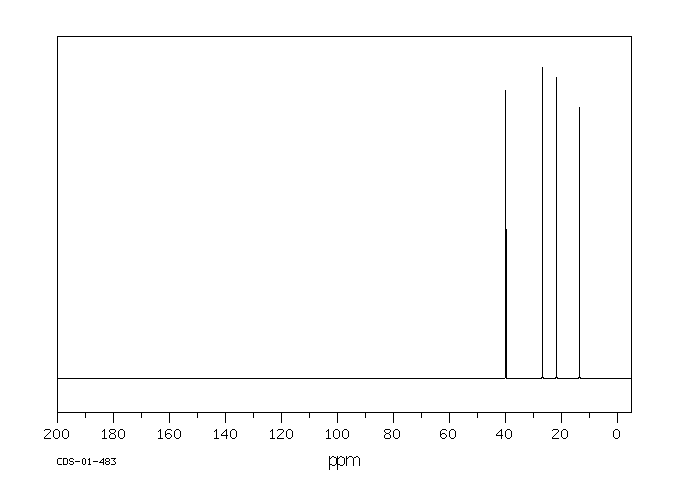
碳谱13CNMR
-
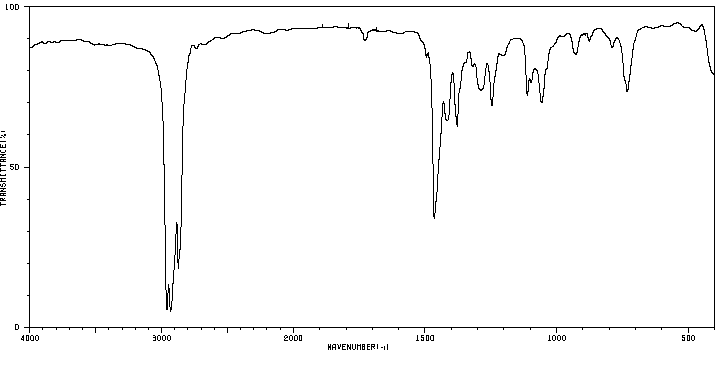
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
黄酸异丁酯
黃原醯胺
高胱胺
香菇素
颜料红88
颜料紫36
顺式-2-异硫氰基-1-环戊羧酸乙酯
顺式-1,2-二(乙硫基)-1-丙烯
顺-2-异硫氰基-1-环己烷羧酸乙酯
非班太尔-D6
雷西那得中间体
雷尼替丁EP杂质J
降钙素杂质15
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
镉离子通道 I
锍,甲基羰基-
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
铯硫氰酸盐
铜,丙烷-2-硫醇
铊(I)2-甲基丁烷-2-硫醇盐
铅离子载体III
铅,丙烷-1-硫醇
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
钠二乙基氨基二硫代甲酸盐三水合物
钠2-羟基乙基碳o三硫代酸酯
钌(3+)三氯化-亚硫酰基二甲烷(1:2)氨酸酯
金刚烷-2-硫酮
野麦畏
酸性橙G
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛烷,1,1,1,2,2,3,3,4,4,5,5,6,6-十三氟-8-[[2-(2-丙烯氧基)乙基]硫代]-
辛基甲烷硫代磺酸酯
辛基甲烷硫代磺酸酯