三苯基胍 | 603-53-2
中文名称
三苯基胍
中文别名
——
英文名称
N,N,N'-triphenyl-guanidine
英文别名
N,N,N'-triphenyl-guanidine;N,N,N'-Triphenyl-guanidin;β-Triphenylguanidin;1,1,3-triphenyl-guanidine;Triphenylguanidin;Triphenyl guanidine;1,1,2-triphenylguanidine
CAS
603-53-2
化学式
C19H17N3
mdl
——
分子量
287.364
InChiKey
RYCFRVNAZOREPC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:413.5±28.0 °C(Predicted)
-
密度:1.08±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:22
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:41.6
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三苯基胍 1,2,3-triphenylguanidine 101-01-9 C19H17N3 287.364
反应信息
-
作为反应物:参考文献:名称:Klingner, Hoppe-Seyler's Zeitschrift fur Physiologische Chemie, 1926, vol. 155, p. 238摘要:DOI:
-
作为产物:参考文献:名称:LAPAROSCOPIC TREATMENT OF PEDIATRIC VARICOCELE: A MULTICENTER STUDY OF THE ITALIAN SOCIETY OF VIDEO SURGERY IN INFANCY摘要:Purpose: We report preliminary results of a multicenter study of the Italian Society of Video Surgery in Infancy on the laparoscopic treatment of pediatric varicocele.Materials and Methods: A total of 161 children 6 to 16 years old (median age 12.5) underwent laparoscopic treatment of varicocele at 6 pediatric surgery divisions. Varicocele was on the left side in 159 cases (98.7%) and bilateral in 2 (1.3%), Two boys had recurrent left varicocele, All children were treated with laparoscopy, including ligation of the spermatic veins only in 28 (17.3%), and ligation of the testicular veins and artery in 133 (82.7%). In 10 boys (6.2%) an additional procedure was done simultaneously, including closure of an apparently patent peritoneal vaginal duct on the right side in 7 and resection of epiploic adhesions between the intestinal loops and abdominal wall from previous appendectomy in the remaining 3.Results: Average operative time was 30 minutes and hospitalization was about 24 hours. At followup there were 13 minor complications (8%), including left hydrocele in 9 children who underwent the Palomo technique, minor scrotal emphysema in 2 and umbilical granuloma in 2. In our series varicocele recurred in 1 boy (3.5%) who underwent ligation of the spermatic veins only and in 3 (2.2%) treated with the Palomo technique.Conclusions: Our preliminary experience shows that the results of the laparoscopic approach are comparable to those of the open approach. However, the important advantages of laparoscopy over the open approach are its minimal invasiveness and precision of intervention. Moreover, laparoscopy allows treatment of other intra-abdominal pathological conditions using the same anesthesia, as in 10 patients in our series. We believe that ligating the testicular veins and artery is preferable to ligating the testicular veins only, even if the incidence of hydrocele is not negligible after the Palomo procedure.DOI:10.1016/s0022-5347(05)67604-5
文献信息
-
Processes for preparing functionahzed polymers, related functionalizing compound and preparation thereof申请人:Bridgestone Corporation公开号:US10730961B2公开(公告)日:2020-08-04Disclosed herein are processes for preparing functionalized polymers, functionalizing compounds useful in the processes and processes for preparing the functionalizing compound. The processes for preparing a functionalized polymer include reaction of a functionalizing compound (prepared from the reaction of an alkoxysilane compound of formula (I) with a polyol of formula (II)) with a reactive conjugated diene monomer-containing polymer, thereby producing a polymer end functionalized with the functionalizing compound. In certain embodiments, the functionalizing compound has a formula according to formula (III), (IV), or (V).公开了用于制备官能化聚合物的过程、在过程中有用的官能化化合物以及制备官能化化合物的过程。制备官能化聚合物的过程包括使官能化化合物(由公式(I)的烷氧基硅烷化合物与公式(II)的多羟基醇反应制得)与包含反应性共轭二烯单体聚合物反应,从而产生以官能化化合物端基官能化的聚合物。在某些实施方式中,官能化化合物的公式符合公式(III)、(IV)或(V)。
-
Compounding silica-reinforced rubber with low volatile organic compound (VOC) emission申请人:Hergenrother L. William公开号:US20060217473A1公开(公告)日:2006-09-28Alkoxy-modified silsesquioxane compounds are described. The alkoxy-modified silsesquioxane compounds contain an alkoxysilane group that participates in an alkoxysilane-silica reaction as a silica dispersing agent in rubber, with the release of zero to about 0.1% by weight of the rubber of volatile organic compounds (VOC), especially alcohol, during compounding and further processing. Further described are methods for making alkoxy-modified silsesquioxanes, methods for making vulcanizable rubber compounds containing alkoxy-modified silsesquioxanes, vulcanizable rubber compounds containing alkoxy-modified silsesquioxanes, and pneumatic tires comprising a component that contains alkoxy-modified silsesquioxanes.
-
NOVEL CARBAMATE ESTER COMPOUND AND ACRYLIC RUBBER COMPOSITION CONTAINING THE SAME申请人:UNIMATEC CO., LTD.公开号:US20220009934A1公开(公告)日:2022-01-13A carbamate ester compound represented by the general formula: Z—OCONH(CH 2 ) n NHCOO—Z [I] wherein Z is [i], [ii], or [iii] below, and n is an integer of 2 to 10, (wherein R 1 and R 2 are each independently a lower alkyl group having 1 to 5 carbon atoms, R 3 is a hydrogen atom or a lower alkyl group having 1 to 5 carbon atoms, and a is 1 or 2). The carbamate ester compound is used as a vulcanizing agent for carboxyl group-containing acrylic rubber and improves the delay of the vulcanization rate by scorch suppression.
-
Phenylurethane compounds and methods for producing same, asymmetric urea compounds and methods for producing same, barbituric acid derivative, and diazo thermal recording material containing the derivative申请人:FUJI PHOTO FILM CO., LTD.公开号:US20020161225A1公开(公告)日:2002-10-31Phenylurethane compounds of the following general formula (1); asymmetric urea compounds of the following general formula (10) obtained from the phenylurethane compounds; barbituric acid derivatives of general formula (18) produced from the asymmetric urea compounds, which have specific substituents and are useful in diazo thermal recording materials; and diazo thermal recording materials containing the barbituric acid derivative. 1
-
Layered product and medical supply comprising the layered product申请人:——公开号:US20040241478A1公开(公告)日:2004-12-02A laminate comprising at least a surface layer (I) and an adjacent layer (II) thereto, characterized in that the surface layer (I) comprises a cross-linked copolymer formed by cross-linking a copolymer prepared by using as monomers at least one vinyl aromatic compound and at least one conjugated diene compound, and the adjacent layer (II) comprises a hydrogenated copolymer formed by hydrogenating a copolymer prepared by using as monomers at least one vinyl aromatic compound and at least one conjugated diene compound; a multilayer tube comprising the laminate; and a medical device comprising the laminate, and/or the multilayer tube.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
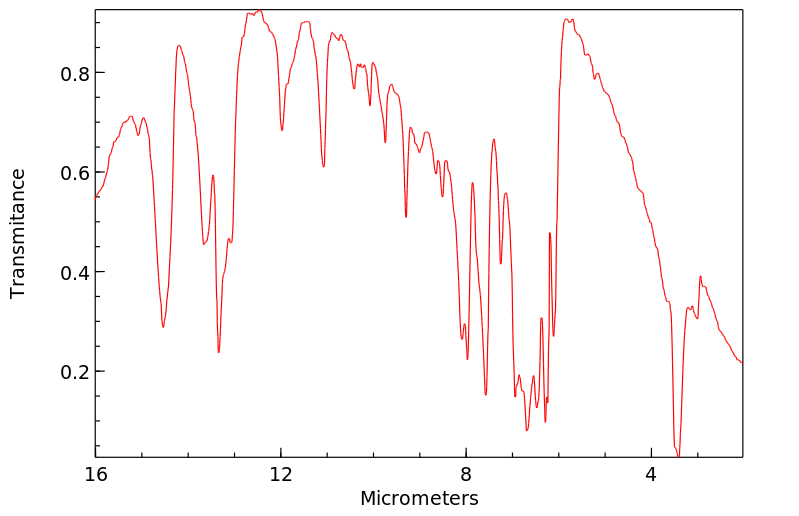
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫