二异丙基二氯硅烷 | 7751-38-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:<0°C
-
沸点:66 °C27 mm Hg(lit.)
-
密度:1.026 g/mL at 20 °C(lit.)
-
闪点:43°C
-
溶解度:溶解大多数有机溶剂。
-
稳定性/保质期:
具有强烈腐蚀性,使用时需佩戴防护器具。
计算性质
-
辛醇/水分配系数(LogP):2.96
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:No
-
危险等级:3/8
-
危险品标志:C
-
安全说明:S28,S36/37/39,S45
-
危险类别码:R10
-
WGK Germany:3
-
海关编码:29319090
-
危险品运输编号:UN 2986 8/PG 2
-
危险标志:GHS02,GHS05
-
危险性描述:H226,H314
-
危险性防范说明:P280,P305 + P351 + P338,P310
-
储存条件:存放于惰性气体中,并避免接触湿气(以免发生分解)。
SDS
模块 1. 化学品
产品名称: Dichlorodiisopropylsilane
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
只可存放于原用的容器内。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
二异丙基二氯硅烷 修改号码:2
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 二异丙基二氯硅烷
百分比: >98.0%(GC)
CAS编码: 7751-38-4
分子式: C6H14Cl2Si
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,二氧化碳
不适用的灭火剂: 水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却,注意切勿直接接触水。如果安全,消除一切火源
。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 切勿与水接触。移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防
爆设备。
模块 7. 操作处置与储存
处理
二异丙基二氯硅烷 修改号码:2
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
可能产生高压。小心打开。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放于惰性气体环境中。
防湿。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅红黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 170 °C
闪点: 43°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
蒸气密度: >1
密度: 1.03
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 与水接触分解并产生有毒气体。
避免接触的条件: 潮敏
须避免接触的物质 氧化剂, 碱, 水
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢, 氧化硅
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
二异丙基二氯硅烷 修改号码:2
模块 11. 毒理学信息
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
副危险性: 第8类 腐蚀品
UN编号: 2985
正式运输名称: 氯硅烷, 易燃的, 腐蚀的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:Preparation and properties of perisopropylcyclotetrasilane, [(Me2CH)2Si]4摘要:DOI:10.1016/s0022-328x(00)85824-6
-
作为产物:参考文献:名称:Production processes for triorganomonoalkoxysilanes and triorganomonochlorosilanes摘要:含有庞大烃基或基团R的硅烷,其具有式(III)R3-(x+y)(R1)x(R2)ySi(OR3),可通过反应式(I)(R1)x(R2)ySiCl3-(x+y)(OR3)的硅烷与式(II)RMgX的格氏试剂制得。此外,式(XIIa)(R1)(R2)(R3)SiCl的三有机氯硅烷可通过反应式(XIa)(R1)(R2)(R3)SiZ1的三有机硅烷与盐酸制得。再者,当式(XXI)(R1)x(R2)ySiCl4-(x+y)的硅烷与式(XXII)RMgX的格氏试剂反应,并在反应过程中加入并反应酒精或环氧化合物时,可制得式(XXIII)R3-(x+y)(R1)x(R2)ySi(OR3)的三有机单烷氧基硅烷。公开号:US20050070730A1
-
作为试剂:描述:3-O-tert-butyldimethylsilyl-L-rhamnal 、 毛地黄毒苷配基 在 二异丙基二氯硅烷 、 MPEG-resin 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 4 A molecular sieve 、 camphor-10-sulfonic acid 、 氟化铵 、 四丁基氟化铵 作用下, 以 二氯甲烷 、 四氢呋喃 为溶剂, 反应 96.0h, 生成 4-[(3S,5R,8R,9S,10S,13R,14S,17R)-3-((2S,4S,5R,6S)-4,5-Dihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-14-hydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 、 4-[(3S,5R,8R,9S,10S,13R,14S,17R)-3-((2R,4S,5R,6S)-4,5-Dihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-14-hydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one参考文献:名称:Jesberger; Jaunzems; Jung, Synlett, 2000, # 9, p. 1289 - 1293摘要:DOI:
文献信息
-
Palladium‐Catalyzed Synthesis of Benzophenanthrosilines by C−H/C−H Coupling through 1,4‐Palladium Migration/Alkene Stereoisomerization作者:Tomohiro Tsuda、Yuka Kawakami、Seung‐Min Choi、Ryo ShintaniDOI:10.1002/anie.202000217日期:2020.5.18catalysis. The reaction mechanism has been experimentally investigated and a catalytic cycle involving a C-H/C-H coupling through a new mode of 1,4-palladium migration with concomitant alkene stereoisomerization has been proposed.
-
ASYMMETRIC BIFUNCTIONAL SILYL MONOMERS AND PARTICLES THEREOF AS PRODRUGS AND DELIVERY VEHICLES FOR PHARMACEUTICAL, CHEMICAL AND BIOLOGICAL AGENTS申请人:The University of North Carolina at Chapel Hill公开号:US20170021030A1公开(公告)日:2017-01-26Asymmetric bifunctional silyl (ABS) monomers comprising covalently linked pharmaceutical, chemical and biological agents are described. These agents can also be covalently bound via the silyl group to delivery vehicles for delivering the agents to desired targets or areas. Also described are delivery vehicles which contain ABS monomers comprising covalently linked agents and to vehicles that are covalently linked to the ABS monomers. The silyl modifications described herein can modify properties of the agents and vehicles, thereby providing desired solubility, stability, hydrophobicity and targeting.
-
Novel Silyl Ether-Based Acid-Cleavable Antibody-MMAE Conjugates with Appropriate Stability and Efficacy作者:Yanming Wang、Shiyong Fan、Dian Xiao、Fei Xie、Wei Li、Wu Zhong、Xinbo ZhouDOI:10.3390/cancers11070957日期:——
Antibody-drug conjugate (ADC) is a novel efficient drug delivery system that has been successfully used in clinical practice, and it has become a research hotspot in the anti-tumor drug field. Acid-cleavable linkers were first used in clinical ADCs, but their structural variety (e.g., hydrazone and carbonate) is still limited, and their stability is usually insufficient. Designing novel acid-cleavable linkers for the conjugation of the popular cytotoxin monomethyl auristatin E (MMAE) has always been a significant topic. In this paper, we generate a novel, silyl ether-based acid-cleavable antibody-MMAE conjugate, which skillfully achieves efficient combination of amino-conjugated MMAE with the acid-triggered silyl ether group by introducing p-hydroxybenzyl alcohol (PHB). The stability, acid-dependence cleavage, effective mechanism, efficacy and safety of the resulting ADC were systematically studied; the results show that it exhibits a significant improvement in stability, while maintaining appropriate efficacy and controlled therapeutic toxicity. This strategy is expected to expand a new type of acid-cleavable linkers for the development of ADCs with highly potent payloads.
抗体药物偶联物(ADC)是一种新颖的高效药物传递系统,已在临床实践中成功使用,并成为抗肿瘤药物领域的研究热点。酸性切割连接剂最初被用于临床ADCs,但它们的结构多样性(例如,腙和碳酸酯)仍然有限,而且它们的稳定性通常不足。设计新型酸性切割连接剂用于将流行的细胞毒素单甲基奥里司亭E(MMAE)进行偶联一直是一个重要的课题。在本文中,我们生成了一种新型的、基于硅醚的酸性切割抗体-MMAE偶联物,它巧妙地通过引入对羟基苄醇(PHB)实现了氨基偶联MMAE与酸性触发硅醚基团的 有效结合。研究了所得ADC的稳定性、酸性依赖切割、有效机制、疗效和安全性;结果表明,它在大稳定性方面有显著改善,同时保持了适当的疗效和可控的治疗毒性。这种策略有望为开发具有高活性载荷的ADCs扩展新型酸性切割连接剂。 -
Low-Coordinate Germylene and Stannylene Heterocycles featuring Sterically Tunable Bis(amido)silyl Ligands作者:S. M. Ibrahim Al-Rafia、Paul A. Lummis、Michael J. Ferguson、Robert McDonald、Eric RivardDOI:10.1021/ic101485a日期:2010.10.18series of monomeric heterocyclic metallylenes [iPr2Si(NR)2}M:] (M = Ge and Sn; R = Dipp = 2,6-iPr2C6H3 or SiPh3) have been prepared. Preliminary atom-transfer chemistry involving the new low-valent germylenes with the chalcogen sources Me3NO and S8 yielded the corresponding dimeric oxo- and sulfido complexes (e.g., [iPr2Si(NDipp)2}Ge(μ-E)]2; E = O and S). Structural analyses of the metallylenes and已经制备了一系列单体杂环金属亚甲基[ i Pr 2 Si(NR)2 } M:](M = Ge和Sn; R = Dipp = 2,6 - iPr 2 C 6 H 3或SiPh 3)。初步的原子转移化学包括具有硫族元素源Me 3 NO和S 8的新的低价亚甲基苯,生成了相应的二聚氧-硫键配合物(例如[ i Pr 2 Si(NDipp)2 } Ge(μ-E )] 2; E = O和S)。对金属亚甲基及其氧化产物的结构分析表明,在NSiN螯合物中并入伞形三芳基甲硅烷基(SiPh 3)相对于Dipp基团,对第14基团中心提供了额外的空间保护。将可空间修饰的-SiAr 3(Ar =芳基)单元作为双(酰胺基)配体阵列的一部分包括在内,代表了该领域的一种新方法,并且在实现越来越高的空间体积方面具有可观的前景。
-
Synthesis of Epoxyquinol A and Related Molecules: Probing Chemical Reactivity of Epoxyquinol Dimers and 2<i>H</i>-Pyran Precursors作者:Chaomin Li、John A. PorcoDOI:10.1021/jo050897o日期:2005.7.1[4 + 4] dimerization of 2H-pyran epoxyquinol monomers. Modifications of 2H-pyran precursors have been explored, including alteration of epoxy alcohol and diene stereochemistry. A stable 2H-pyran prepared by alteration of the epoxyquinol 2H-pyran nucleus was evaluated as a diene in Diels−Alder cycloaddition with reactive dienophiles. Extensive studies for improving the [4 + 4] dimerization of selectively使用2 H-吡喃环氧喹啉单体的[4 + 2]和[4 + 4]二聚作用,完成了环氧喹啉二聚体,环氧喹啉A,B和环氧双醇A(RKB-3564 D)的总合成。已经研究了2 H-吡喃前体的修饰,包括环氧醇的改变和二烯的立体化学。通过改变环氧喹诺2 H-吡喃核制备的稳定的2 H-吡喃在Diels-Alder环加成反应性亲二烯体中被评估为二烯。改善选择性保护2 H的[4 + 4]二聚化的广泛研究-吡喃单体可提供新型环氧醌类二聚体环氧双醇A,并获得了关于竞争性[4 + 2]和[4 + 4]二聚过程的宝贵见解。此外,已评估了环氧喹诺尔二聚体的化学反应性和结构修饰,包括[2 + 2]光环加成和[3,3]σ重排,表明有可能从二聚环氧醌类天然产物骨架中产生新的结构多样性。
表征谱图
-
氢谱1HNMR
-
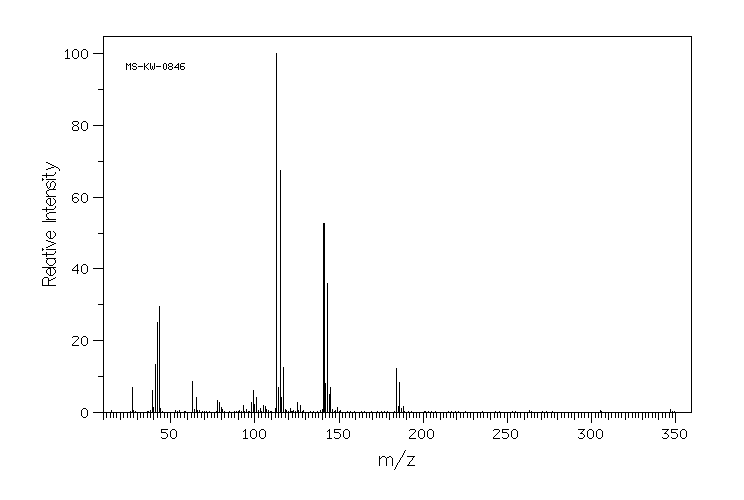
质谱MS
-
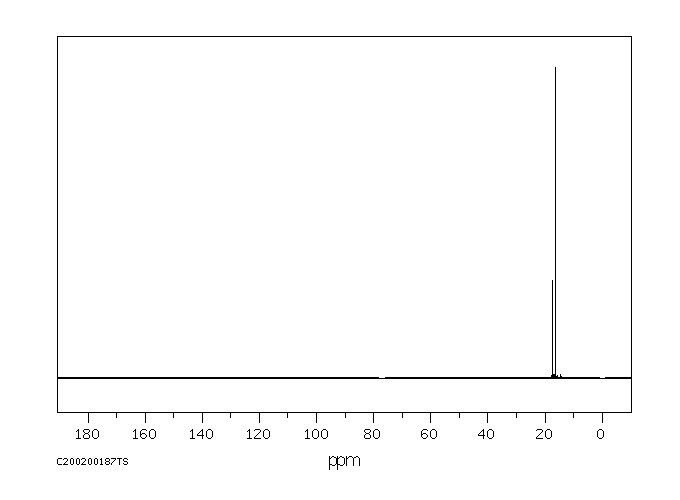
碳谱13CNMR
-
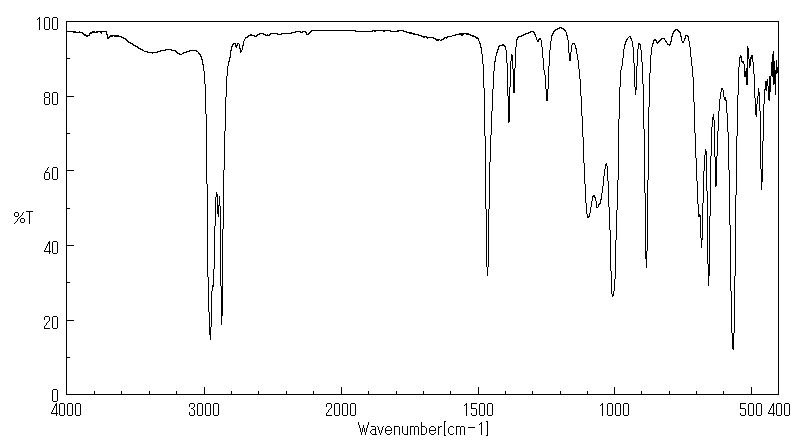
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息