(2-丁基-苯氧基)-乙酸 | 102167-36-2
中文名称
(2-丁基-苯氧基)-乙酸
中文别名
——
英文名称
(2-Butyl-phenoxy)-essigsaeure
英文别名
<2-Butyl-phenoxy>-essigsaeure;(2-butyl-phenoxy)-acetic acid;O-(2-Butyl-phenyl)-glykolsaeure;(O-Butylphenoxy)acetic acid;2-(2-butylphenoxy)acetic acid
CAS
102167-36-2
化学式
C12H16O3
mdl
——
分子量
208.257
InChiKey
APIOACZJBZKBFB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:105 °C
-
沸点:325.9±17.0 °C(Predicted)
-
密度:1.093±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:15
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.42
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
上下游信息
反应信息
-
作为反应物:参考文献:名称:Kulkarni et al., Journal of the Karnatak University, 1957, vol. 2, p. 69,70, 76摘要:DOI:
-
作为产物:描述:参考文献:名称:818.酸催化的烷基芳基醚的重排。第一部分。丁基苯醚与氯化铝的重排摘要:DOI:10.1039/jr9590004080
文献信息
-
Peroxyacids, phenoxyacetate peracid precursors and perhydrolysis system申请人:The Clorox Company公开号:EP0267048A2公开(公告)日:1988-05-11A perhydrolysis system for in situ generation of peroxyacid characterised in that it comprises: a peroxyacid percursor including at least one α-substituted carbonyl moiety having the structure: wherein R is hydrogen or an alkyl with not more than 5 carbons and L is a group whose conjugate acid has a pKa in aqueous solution of from about 5 to about 13; and a source of peroxygen which will react with the peroxyacid precursor in aqueous solution to form a peroxyacid having the structure: is disclosed. Peroxyacids and peroxyacid precursors are also disclosed.
-
Pigment epithelial detachment in the elderly作者:D. Pauleikhoff、D. Löffert、G. Spital、M. Radermacher、J. Dohrmann、A. Lommatzsch、A. BirdDOI:10.1007/s00417-002-0505-8日期:2002.7Background: A prospective analysis was performed to characterize the angiographic appearance, natural course and prognosis of serous pigment epithelial detachments (PEDs) in elderly patients. The aim was to differentiate PEDs according to their angiographic characteristics and to analyze the specific clinical, visual and morphologic course of the different PEDs. Methods: Fluorescein and indocyanine green angiography were performed in 10 1 consecutive patients (53-87 years; 63 female, 38 male) with clinical signs of serous PED and drusen. Results: Different types of serous PED were identified: polypoidal choroidal vasculopathy (PCV)-associated PED in 14 patients (13.9%), vascular PED in 72 (71.2%), and avascular PED in 15 (14.9%). All PEDs resulted initially in similar visual loss. Avascular PEDs were smaller than vascular PEDs, and the latter were smaller than PCV-PEDs. During follow-up these differences were always present, but all PEDs enlarged initially followed by regression. This course was associated in all PEDs with progressive visual loss, accompanied by the development of RPE atrophy in avascular PEDs or disciform scars or RPE tears in the two other types. Conclusion: Despite different associations, all PEDs have a similar clinical course with respect to visual loss and enlargement or regression. This is compatible with the proposed common pathogenetic background with a hydrophobic barrier in Bruch's membrane causing fluid resulting from RPE pumping activity to accumulate between the pigment epithelium and Bruch's membrane.
-
HETEROCYCLIC COMPOUNDS AS PLATELET AGGREGATION INHIBITORS申请人:ZENECA LIMITED公开号:EP0690847A1公开(公告)日:1996-01-10
-
SUBSTITUTED 1,3,5-TRIAZINES AND PYRIMIDINES AS ABCA-1 ELEVATING COMPOUNDS AGAINST CORONARY ARTERY DISEASE OR ATHEROSCLEROSIS申请人:CV THERAPEUTICS, INC.公开号:EP1341773A2公开(公告)日:2003-09-10
-
METHOD OF OLIGONUCLEOTIDE LABELING USING CYCLOADDITION REACTION申请人:Chemgenes Corporation公开号:EP2427573A2公开(公告)日:2012-03-14
表征谱图
-
氢谱1HNMR
-
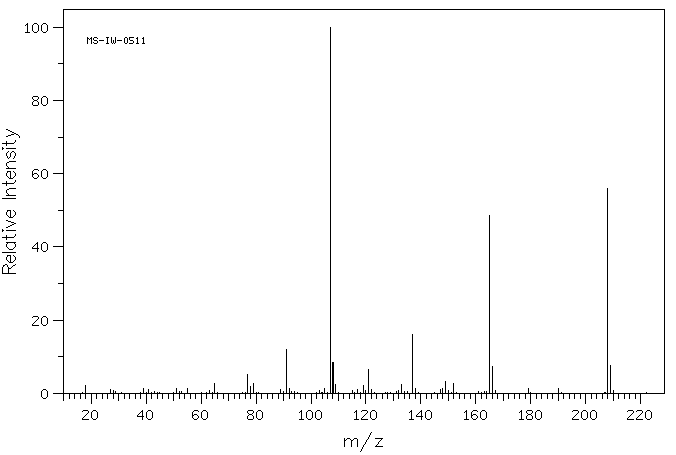
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫