1,2,5,6-四溴己烷 | 58443-86-0
中文名称
1,2,5,6-四溴己烷
中文别名
1,2,5,6-四溴己烷(非对映异构体混合物)
英文名称
1,2,5,6-tetrabromohexane
英文别名
1,2,5,6-Tetrabrom-hexan
CAS
58443-86-0
化学式
C6H10Br4
mdl
——
分子量
401.761
InChiKey
WPBWUVCMCYXPFI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:47.0 to 51.0 °C
-
稳定性/保质期:
对水没有危害,请勿在未获得政府许可的情况下将材料排放到周围环境中。
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险类别码:R20/21/22
-
安全说明:S23,S36
-
储存条件:将贮存于密封容器中,并置于干燥、阴凉的地方保存。
SDS
1,2,5,6-Tetrabromohexane (mixture of Revision number: 5
diastereoisomers)
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,2,5,6-Tetrabromohexane (mixture of diastereoisomers)
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Warning
Signal word
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
1,2,5,6-Tetrabromohexane (mixture of diastereoisomers)
Components:
Percent: >97.0%(GC)
58443-86-0
CAS Number:
Chemical Formula: C6H10Br4
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
1,2,5,6-Tetrabromohexane (mixture of
diastereoisomers)
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
pH: No data available
1,2,5,6-Tetrabromohexane (mixture of
diastereoisomers)
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Melting point/freezing point:49°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents, Strong bases
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
1,2,5,6-Tetrabromohexane (mixture of
diastereoisomers)
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
diastereoisomers)
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,2,5,6-Tetrabromohexane (mixture of diastereoisomers)
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Warning
Signal word
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
1,2,5,6-Tetrabromohexane (mixture of diastereoisomers)
Components:
Percent: >97.0%(GC)
58443-86-0
CAS Number:
Chemical Formula: C6H10Br4
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
1,2,5,6-Tetrabromohexane (mixture of
diastereoisomers)
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
pH: No data available
1,2,5,6-Tetrabromohexane (mixture of
diastereoisomers)
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Melting point/freezing point:49°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents, Strong bases
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
1,2,5,6-Tetrabromohexane (mixture of
diastereoisomers)
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
电池材料
反应信息
-
作为反应物:描述:1,2,5,6-四溴己烷 在 叔丁基锂 、 potassium carbonate 作用下, 以 乙醚 、 乙醇 、 正戊烷 为溶剂, 反应 93.8h, 生成 1,4,6,9-tetramethylene-5-silaspiro[4.4]nonane参考文献:名称:稳定全碳取代的甲硅烷基的途中:双(α-螺环丙基)甲硅烷基的合成与反应性摘要:报道了双(α-螺环丙基)亚甲硅烷基的合成及其反应性。通过UV光介导的三硅烷前体的光解来完成亚甲硅烷基的解放。插入和加成反应证明了这个新的双(α-螺环丙基)取代的亚甲硅烷基家族的存在和多功能性。在侧翼环丙基上进行取代以改善反应中心的空间屏蔽仍然具有挑战性。DOI:10.1021/om500485m
-
作为产物:参考文献:名称:稳定全碳取代的甲硅烷基的途中:双(α-螺环丙基)甲硅烷基的合成与反应性摘要:报道了双(α-螺环丙基)亚甲硅烷基的合成及其反应性。通过UV光介导的三硅烷前体的光解来完成亚甲硅烷基的解放。插入和加成反应证明了这个新的双(α-螺环丙基)取代的亚甲硅烷基家族的存在和多功能性。在侧翼环丙基上进行取代以改善反应中心的空间屏蔽仍然具有挑战性。DOI:10.1021/om500485m
文献信息
-
Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations作者:Xichang Dong、Johannes L. Roeckl、Siegfried R. Waldvogel、Bill MorandiDOI:10.1126/science.abf2974日期:2021.1.29shuttle (e-shuttle) paradigm for the facile and scalable interconversion of alkenes and vicinal dihalides, a class of reactions that can be used both to synthesize useful dihalogenated molecules from simple alkenes and to recycle waste material through retro-dihalogenation. The reaction is demonstrated using 1,2-dibromoethane, as well as 1,1,1,2-tetrachloroethane or 1,2-dichloroethane, to dibrominate or
-
Enantioselective Total Synthesis of (+)-Testudinariol A Using a New Nickel-Catalyzed Allenyl Aldehyde Cyclization作者:Kande K. D. Amarasinghe、John MontgomeryDOI:10.1021/ja027148y日期:2002.8.1An enantioselective total synthesis of (+)-testudinariol A was completed. A new nickel-catalyzed allenyl aldehyde cyclization was developed in the approach. In addition, an asymmetric anti aldol reaction and a two-directional oxocarbenium ion/vinyl silane condensation were employed as key steps.
-
An Electron-Catalyzed Cope Cyclization. The Structure of the 2,5-Dicyano-1,5-hexadiene Radical Anion in the Gas Phase作者:Loubna A. Hammad、Paul G. WentholdDOI:10.1021/ja037143g日期:2003.9.1precursor ion. Attempts to generate radical anions of acrylonitrile and 2,6-dicyano-1,6-heptadiene were unsuccessful, providing additional evidence against a linear structure as a stable structure for 2,5-dicyano-1,5-hexadiene radical anion. The cyclization of the radical anion of the 2,5-dicyano-1,5-hexadiene is the first example of an electron-catalyzed Cope cyclization.2,5-二氰基-1,5-己二烯的自由基阴离子显示在流动的余辉-三重四极杆装置中进行科普环化。2,5-二氰基-1,5-己二烯自由基阴离子的环状结构是利用化学反应性建立的。离子通过加成与 CO2 和 CS2 反应,而闭壳分子(如富马腈)的自由基阴离子不与这些试剂反应。该离子表现出异张离子的反应性特征,因为它依次添加 和 NO 或 NO2。它通过形成 m/z 135 的产物与 NO 反应,对应于添加,然后失去 HCN。产物离子在 m/z 135 处的反应性和 CID 谱与需要环状母离子的肟酸根离子的反应性和 CID 谱一致。尝试生成丙烯腈和 2,6-二氰基-1,6-庚二烯的自由基阴离子未成功,提供额外的证据反对线性结构作为 2,5-二氰基-1,5-己二烯自由基阴离子的稳定结构。2,5-二氰基-1,5-己二烯的自由基阴离子的环化是电子催化科普环化的第一个例子。
-
Experimental Acute Necrotising Pancreatitis: Evaluation and Characterisation of a Model of Intraparenchymal Injection of Sodium Taurocholate in Rats作者:Haim ParanDOI:10.1080/110241500447308日期:2000.10.20ObjectivesTo evaluate a simple model that produces progressive dose dependent pancreatitis, by intraparenchymal injection of sodium taurocholate.DesignOpen laboratory study.SettingTeaching hospital, Israel.MaterialsForty eight Wistar rats.InterventionsSodium taurocholate was injected, 0.3 ml/100 g body weight, in concentrations of 5% and 10% into the pancreatic parenchyma of 32 Wistar rats, resulting in two distinct groups of severity. In 16 sham controls, saline was injected into the pancreas in similar fashion. Blood samples were withdrawn before, and 6, 24, 48, and 72 hours after induction of pancreatitis.ResultsSix hours after taurocholate injection, there was a sharp increase in the plasma activities of amylase, lipase, and lactate dehydrogenase (LDH). After 24 hours plasma activities of amylase and lipase decreased to near normal values while LDH remained slightly increased for 48 hours and decreased only after 72 hours. At 6 hours after the injection, interleukin-6 (IL-6) concentrations had increased slightly in the 5% group and decreased to the baseline values at 24 hours. In the 10% group, the increase in IL-6 values was significantly greater than in the 5% group (p = 0.04), and correlated well with severity of pancreatitis as defined by histology (p = 0.01) and mortality (p = 0.037). Twenty four hours after injection of taurocholate, morphological changes comprising diffuse necrosis of the pancreas, fat necrosis, and intestinal dilatation secondary to paralytic ileus were severe. Histopathological examination of the pancreas showed good correlation with the clinical findings and with mortality.No morphological changes were detected when saline was injected into the pancreas (sham control), and only mild rises of IL-6, lipase, amylase, and LDH activities were seen at 6 hours after injection. The mortality, after 10 days, was 80% in the 10% taurocholate group, 30% in the 5% taurocholate group, and 0 in the sham control group (p < 0.05).ConclusionThe intraparenchymal injection of taurocholate is easy to perform and highly reproducible. The histopathological injury is dose-dependent, as is the mortality. We conclude that this model is valuable for the study of new treatments for pancreatitis.干预措施向 32 只 Wistar 大鼠的胰腺实质注射浓度为 5% 和 10% 的牛磺胆酸钠,每 100 克体重注射 0.3 毫升,结果分为两组不同的严重程度。在 16 个假对照组中,以类似方式向胰腺注射生理盐水。结果注射牛磺胆酸钠 6 小时后,血浆中淀粉酶、脂肪酶和乳酸脱氢酶(LDH)的活性急剧上升。24 小时后,血浆中淀粉酶和脂肪酶的活性降至接近正常值,而 LDH 在 48 小时内仍略有升高,72 小时后才有所下降。注射后 6 小时,5% 组的白细胞介素-6(IL-6)浓度略有上升,24 小时后降至基线值。在10%组中,IL-6值的增加明显高于5%组(p = 0.04),并且与组织学定义的胰腺炎严重程度(p = 0.01)和死亡率(p = 0.037)密切相关。注射牛磺胆酸钠 24 小时后,胰腺弥漫性坏死、脂肪坏死和肠扩张等形态学变化严重,继发于麻痹性回肠。向胰腺注射生理盐水(假对照)时未发现形态学变化,注射后 6 小时仅发现 IL-6、脂肪酶、淀粉酶和 LDH 活性轻度升高。10 天后,10% 牛磺胆酸盐组的死亡率为 80%,5% 牛磺胆酸盐组为 30%,假对照组为 0(P < 0.05)。组织病理学损伤与死亡率一样,都与剂量有关。我们认为,该模型对研究胰腺炎的新疗法很有价值。
-
Thermal Rearrangements, 28. Competing Reaction Pathways in the Thermal Cycloisomerization of 1,3-Hexadien-5-ynes作者:Uta Nüchter、Gerhard Zimmermann、Viola Francke、Henning HopfDOI:10.1002/jlac.199719970729日期:1997.7The thermal cycloisomerization of the 1,3-hexadiene-5-ynes 1a–e has been studied at temperatures above 500°C and reaction times of about 0.3 s (1a–d) and at a pressure of 1.5 · 10−2 Torr (FVP, 1e), respectively, at low partial pressures in quartz flow systems. While 1a and 1b cycloisomerize exclusively to the corresponding benzenes and pentafulvenes, substrates 1c–e cyclize primarily to 4-substituted
表征谱图
-
氢谱1HNMR
-
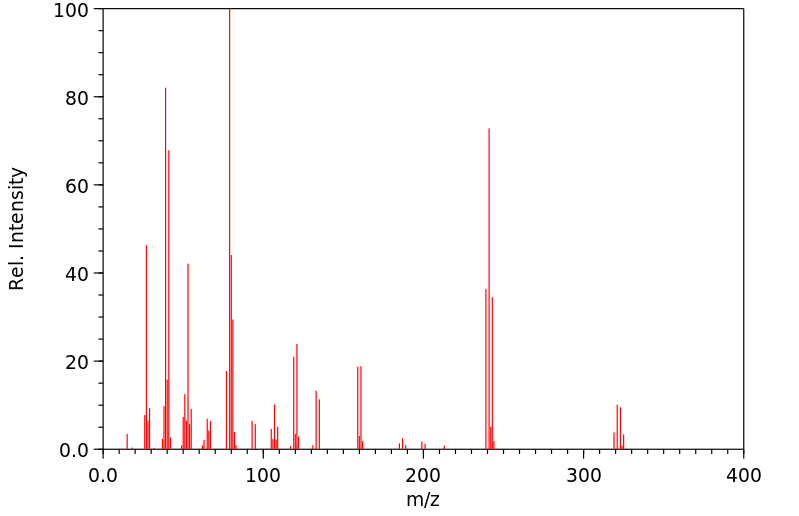
质谱MS
-
碳谱13CNMR
-
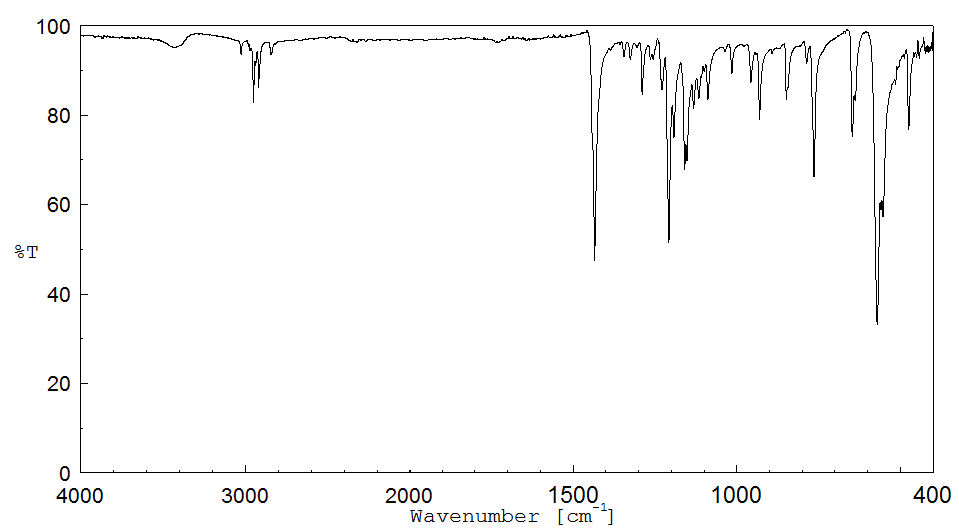
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4