1,4-二甲氧基蒽 | 13076-29-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:134.5-136.5 °C
-
沸点:340.88°C (rough estimate)
-
密度:1.0981 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:18
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
海关编码:2909309090
-
储存条件:存储于阴凉干燥处。
SDS
| Name: | 1 4-Dimethoxyantracene 98% (uv-vis) Material Safety Data Sheet |
| Synonym: | |
| CAS: | 13076-29-4 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 13076-29-4 | Anthracene, 1,4-dimethoxy- | 98.0 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 13076-29-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 134.50 - 136.50 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C16H14O2
Molecular Weight: 238.29
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 13076-29-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Anthracene, 1,4-dimethoxy- - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 13076-29-4: No information available.
Canada
CAS# 13076-29-4 is listed on Canada's NDSL List.
CAS# 13076-29-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 13076-29-4 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1,4-dihydroxyanthracene 7218-35-1 C14H10O2 210.232
反应信息
-
作为反应物:描述:1,4-二甲氧基蒽 在 氢氧化钾 作用下, 以 乙醇 为溶剂, 反应 10.0h, 生成 1,4-dimethoxy-9,10-dihydro-9,10-ethanoanthracene-11,12-diol参考文献:名称:Antitumor agents. II. Bis(guanylhydrazones) of anthracene-9,10-dicarboxaldehydes摘要:9,10-Anthracenedicarboxaldehyde bis[(4,5-dihydro-1H-imidazol-2-yl)hydrazone] (bisantrene, VI-1) showed anticancer activity in mice vs. both leukemias and solid tumors. Increases in life span vs. the following neoplasms were: P-388 leukemia, 137%; B-16 melanoma, 122%; Lieberman plasma cell tumor, greater than 85%; colon tumor 26, 150%; Ridgway osteogenic sarcoma, 85%. There were significant numbers of long-term survivors. Both DNA and RNA synthesis were strongly inhibited. The drug was resistant to biodegradation and was bound strongly to tissues; in monkeys the half-life for disappearance from serum was 6 days. Related hydrazones were synthesized, and structure-activity relationships are discussed. Two routes to ring-substituted anthracene-9,10-dicarboxaldehyde intermediates were developed.DOI:10.1021/jm00347a006
-
作为产物:描述:参考文献:名称:Conversion of 9,10-anthraquinones to anthracenes摘要:DOI:10.1021/jo00920a007
-
作为试剂:参考文献:名称:包含电子给体-受体激基复合物的杜瓦瓶的异构化摘要:六甲基(杜瓦瓶)(HMDB)的价光异构化是通过芳族单重光敏剂1,4-二氰基苯,1-氰基萘,9-氰基蒽和9,10-二氰基蒽在环己烷溶剂中进行的,其极限量子效率为1.0。芳族敏化剂的荧光猝灭导致激基复合物发射,其与通过异构体六甲基苯(HMB)淬灭所获得的激基复合物发射相同。根据相对寿命和双重淬灭实验,该发射被识别为HMB激基复合物发射。HMDB衍生(“绝热”)发射的相对产率为20–50%,具体取决于HMB激基复合物产物的激发能。联乙酰单重态或三重态或1-氰基萘三重态光敏化均不能成功实现HMDB的异构化。1,4,5二甲基 6-四甲基二甲基-clo [2.20] hexa-2,5-diene-2,3-dicarboxylate在淬灭电子给体荧光团上的化合价异构化,量子效率为0.2。在这种情况下,不以激发态生成芳香价异构体。讨论了控制杜瓦苯的绝热和非绝热异构化效率的因素,包括敏化剂的氧化还原特性,构DOI:10.1016/s0040-4020(01)92388-9
文献信息
-
A New Convenient Synthesis of Alkoxyanthracenes from Alkoxy-9,10-anthraquinones作者:Chuanfeng Chen、Lingang Lu、Qiyin Chen、Xiaozhang ZhuDOI:10.1055/s-2003-42408日期:——yields by zinc in refluxing acetic acid. Under similar conditions, ethyl I'-anthracenoxyacetate (3i) with the ester group unaffected and 1,8-oxybis(ethyleneoxyethyleneoxy)anthracene (5) were also conveniently synthesized in 65 and 70% yields, respectively.
-
Synthesis of a new zinc phthalocyanine–benzoquinone rigid dyad作者:Chi-Hang Lee、Young Ju Yun、Jianchang Guo、Lin X. Chen、Braja. K. MandalDOI:10.1142/s1088424620500510日期:2021.1
A new zinc phthalocyanine–benzoquinone rigid dyad, QnZnPc–G[Formula: see text] was synthesized as a model compound to study photo-induced charge separation mimicking natural photosynthesis. Compared to its previously reported analog, this dyad has an additional fused benzene ring between the zinc phthalocyanine (ZnPc) (donor) and benzoquinone (acceptor) moieties. The rigid structure of QnZnPc–G[Formula: see text] ([Formula: see text] no rotamers) is designed to minimize the unusual electronic perturbation induced by the internal motions, which resulted in a significant increase in the lifetime of the charged separated state (from 40 ps to 252 ps). Physical and photochemical properties of this new dyad were examined and discussed in this paper.
-
Synthesis of Extended Bifunctional Triptycenes作者:Petr Holý、Jiří Rybáček、Jiří ZávadaDOI:10.1055/s-0028-1083209日期:2008.11Extended triptycenes 5a,b and 7a-c have been designed to serve as convenient building blocks for a stepwise ramification of the parent triptycene structure by Diels-Alder oligomerization.
-
Triptycene analogs申请人:——公开号:US20020091163A1公开(公告)日:2002-07-11This invention provides analogs of triptycene which are useful as anticancer drugs, as well as for other uses. The potency of these compounds is in a similar magnitude as daunomycin, a currently used anticancer drug. Each compound of the invention produces one or more desired effects (blocking nucleoside transport, inhibiting nucleic acid or protein syntheses, decreasing the proliferation and viability of cancer cells, inducing DNA fragmentation or retaining their effectiveness against multidrug-resistant tumor cells).这项发明提供了三苯乙烯的类似物,可用作抗癌药物,以及其他用途。这些化合物的效力与目前使用的抗癌药达诺霉素相当。该发明的每种化合物都产生一个或多个期望的效果(阻断核苷酸转运、抑制核酸或蛋白质合成、降低癌细胞的增殖和存活能力、诱导DNA断裂或保持其对多药耐药肿瘤细胞的有效性)。
-
Intramolecular Electron Transfer between Terminal 1,4-Dimethoxybenzene Units in Radical Cations with a [2.2](1,4)Naphthalenophane, [2.2](1,4)Anthracenophane, and Pentacene Skeleton作者:Alexander R. Wartini、Heinz A. Staab、Franz A. NeugebauerDOI:10.1002/(sici)1099-0690(199806)1998:6<1161::aid-ejoc1161>3.0.co;2-1日期:1998.6cations, in which two terminal 1,4-dimethoxybenzene units are anellated to [2.2]paracyclophane (2b•+, 3b•+), [2.2](1,4)naphthalenophane (4d•+), and anthracene bridges (5•+), have been studied by ESR and ENDOR spectroscopy. In the syn- and anti-naphthalenophane radical cations 2b•+ and 3b•+ the delocalization of the unpaired electron over both π-moieties and the distinct difference between the first and各种自由基阳离子,其中两个末端 1,4-二甲氧基苯单元与 [2.2] 对环烷 (2b•+, 3b•+)、[2.2](1,4) 萘烷 (4d•+) 和蒽桥键合 ( 5•+),已通过 ESR 和 ENDOR 光谱研究。在合成和反萘烷自由基阳离子 2b•+ 和 3b•+ 中,未配对电子在两个 π 部分上的离域以及第一和第二氧化电位之间的明显差异,ΔE = E20 – E10,证明了两个电泳之间的大量分子内电子相互作用。通过苯并烷化作用在 4d•+ 和 5 中延伸桥会导致局部自由基阳离子。在 1,4,8,11-四甲氧基-并五苯自由基阳离子 (5•+) 中发现了两种电泳之间的强分子内电子相互作用。描述了 4d 的合成。
表征谱图
-
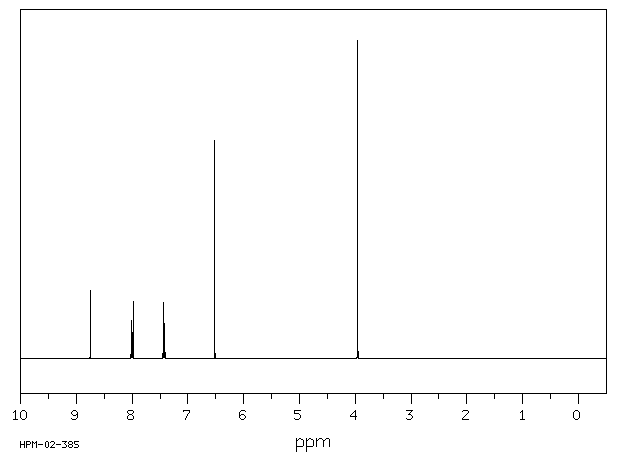
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息