1,4-二甲苯磺酰哌嗪 | 17046-84-3
中文名称
1,4-二甲苯磺酰哌嗪
中文别名
——
英文名称
1,4-bis-(toluene-4-sulfonyl)-piperazine
英文别名
1,4-Bis-(p-toluolsulfonyl)-piperazin;1,4-Ditosylpiperazine;1,4-bis-(4-methylphenyl)sulfonylpiperazine
CAS
17046-84-3
化学式
C18H22N2O4S2
mdl
MFCD00047400
分子量
394.516
InChiKey
LMIXCJFVEQOWSQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:286 °C
-
沸点:565.8±60.0 °C(Predicted)
-
密度:1.334±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:26
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:91.5
-
氢给体数:0
-
氢受体数:6
安全信息
-
海关编码:2933599090
-
储存条件:存储条件:2-8°C,密封,干燥。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-双(乙烯)-对甲苯磺酰胺 ditosylethylenediamine 4403-78-5 C16H20N2O4S2 368.478 N,N-双[2-(对甲苯磺酰氧基)乙基]-对甲苯磺酰胺 N,N-bis(tosyloxyethyl)-p-toluenesulfonamide 16695-22-0 C25H29NO8S3 567.705
反应信息
-
作为反应物:描述:参考文献:名称:硅胶中的碱金属(M-SG):一种用于胺脱硫的新试剂。摘要:描述了一种用于仲胺脱硫的新方法。发现吸收到纳米结构二氧化硅(M-SG)中的碱金属是用于一系列N,N-二取代磺酰胺脱硫的有用的固态试剂。M-SG试剂是室温稳定的自由流动粉末,可保留母体金属的化学反应性,从而降低了使用活性金属的危险性和相关成本。DOI:10.1021/ol8021337
-
作为产物:参考文献:名称:304. Polyamines. Part III. The preparation of unsymmetrical amines of the types NHR·C2H4·NH·C2H4·NH2and NH2·C2H4·NH·C3H6·NH2, and the action of ammonia on di-p-toluenesulphonylbis-(β-chloroethyl)ethylenediamine摘要:DOI:10.1039/jr9370001468
文献信息
-
<i>N</i>-(ω-TOSYLOXYALKYL)PHTHALIMIDES AS REACTIVE GENERAL SYNTHONS FOR INTRODUCING ALKYLAMINO GROUPS AND THEIR APPLICATION FOR THE “SELF-PROLIFERATIVE” SYNTHESIS OF OPEN-CHAIN POLYAMINES作者:Masaaki Iwata、Hiroyoshi KuzuharaDOI:10.1246/cl.1986.369日期:1986.3.5New synthetic routes to N-(ω-tosyloxyalkyl)phthalimides (2) were developed and the synthetic utility of 2 as alkylating reagents was exemplified in the open-chain polyamine synthesis involving the ...
-
Bifunctionalization of α,ω-sulphonamides via kinetically controlled reaction of α,ω-bis(N-nitrososulphonamides)作者:Masaaki Iwata、Hiroyoshi KuzuharaDOI:10.1039/c39850000918日期:——α,ω-Bis(N-nitrososulphonamides) in hot benzene were converted into α,ω-disulphonates and bifunctional α-sulophonate-ω-sulphonamides, in good yields, by Intramolecular N-nitrososulphonamide–sulphonate rearrangement.
-
Winawer, 1934, # 6, p. 11,13作者:WinawerDOI:——日期:——
-
Klamann; Bertsch, Chemische Berichte, 1956, vol. 89, p. 2007,2011作者:Klamann、BertschDOI:——日期:——
-
Singh, Paramjit; Jain, Anupa, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1988, vol. 27, # 1-12, p. 790 - 792作者:Singh, Paramjit、Jain, AnupaDOI:——日期:——
表征谱图
-
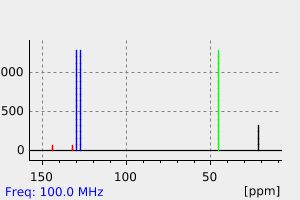
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫