1,9-二氯壬烷 | 821-99-8
中文名称
1,9-二氯壬烷
中文别名
1-十一烯
英文名称
1,9-dichlorononane
英文别名
1,9-Dichlor-nonan
CAS
821-99-8
化学式
C9H18Cl2
mdl
MFCD00001024
分子量
197.148
InChiKey
JMGRNJZUQCEJDB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:258-262 °C(lit.)
-
密度:1.091 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
溶解度:丙酮(微量溶解)、氯仿(可溶)、DMSO(微量溶解)、DMSO(微量溶解)
-
保留指数:1435
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:11
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
安全说明:S26,S37/39
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封储存,应存放在阴凉、干燥的仓库中。
SDS
| Name: | 1 9-Dichlorononane 99% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 821-99-8 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 821-99-8 | 1,9-Dichlorononane | 99 | 212-484-2 |
Risk Phrases: 36/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 821-99-8: Belgium - TWA: (listed as nonane): 200 ppm VLE; 1065 mg/m3 VLE France - VME: (listed as nonane): 200 ppm VME; 1050 mg/m3 VME Germany: (listed as nonane): 200 ppm VME; 1050 mg/m3 VME Japan: (listed as nonane): 200 ppm OEL; 1050 mg/m3 OEL Malaysia: (listed as nonane): 200 ppm TWA; 1050 mg/m3 TWA Netherlands: (listed as nonane): 200 ppm MAC; 1050 mg/m3 MAC Spain: (listed as nonane): 200 ppm VLA-ED; 1065 mg/m3 VLA-ED Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: chlorine-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 258-262 deg C @ 760.00mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 112 deg C (> 233.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.0910g/cm3
Molecular Formula: C9H18Cl2
Molecular Weight: 197.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong bases, strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 821-99-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,9-Dichlorononane - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/38 Irritating to eyes and skin.
Safety Phrases:
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 821-99-8: No information available.
Canada
CAS# 821-99-8 is listed on Canada's NDSL List.
CAS# 821-99-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 821-99-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-氯-9-碘壬烷 1-iodo-9-chlorononane 29215-49-4 C9H18ClI 288.599 11-氯-1-十一碳炔 11-chloro-1-undecyne 29043-93-4 C11H19Cl 186.725 —— 1-chloroheptadec-10-yne 56554-75-7 C17H31Cl 270.886
反应信息
-
作为反应物:参考文献:名称:Synthesis and pharmacological properties of bisquaternary salts of α,ω-Di[N-methyl-N-(1-adamantyl)amino] alkanes摘要:DOI:10.1007/bf00771468
-
作为产物:参考文献:名称:Ahmad; Bumpus; Strong, Journal of the American Chemical Society, 1948, vol. 70, p. 3393摘要:DOI:
文献信息
-
Polycations-12. The Synthesis of Liquid Ionic Phosphates (LIPs) from Mono- and Polycationic Ammonium Halides作者:Robert Engel、JaimeLee Iolani Cohen、Sharon Lall、Valbona Behaj、Danny Mancheno、Robert Casiano、Marie Thomas、Amir Rikin、Jennifer Gaillard、Ravinder Raju、Alexander Scumpia、Steve CastroDOI:10.1055/s-2002-33333日期:——Preparations are reported for an extensive series of phosphate salts of polyammonium species from the corresponding halide salts. The phosphate salts, as anhydrous materials, are liquid at or near room temperature. Three approaches for the preparation of these materials are noted, including treatment with aqueous hexafluorophosphoric acid, classical ion exchange methods, and treatment with 85% phosphoric
-
Experimental and QSAR Studies on Antimicrobial Activity of Benzimidazole Derivatives作者:Ayarivan Puratchikody、Govindasamy Nagalakshmi、Mukesh DobleDOI:10.1248/cpb.56.273日期:——Twenty eight analogues of benzimidazoles had been synthesized and tested for their antimicrobial activity against four bacteria (Staphylococcus auerus, Escherichia coli, Bacillus pumilus and Proteus vulgaris) and two fungi (Aspergillus flavus and Aspergilus niger). Compounds with R as C6H4NO2 and R′ as SO2C6H4–CH3(p), with R as C6H4OCH3 and R′ as SO2C6H4–CH3(p), and with R as CH2C6H5 and R′ as CH2(CH2)9Cl exhibited comparable or higher antibacterial activity than Ciprofloxacin against S. auerus and E. coli and, higher activity than Nystatin against A. flavus. Several other compounds showed better activity than the standard antibiotic for E. coli. Compounds with R as CCl3 and R′ as SO2C6H4–CH3(p) or COC6H5 exhibited the lowest activity against all the organisms. Addition of methylene groups in the R′ position increased activity. Many of the compounds showed better activity than Ciprofloxacin for one or more organisms. Compound with R as CH2OC6H5 and R′ as CH2(CH2)9Cl exhibited higher activity against both the fungii than the control Nystatin. Quantitative structure activity relationships (QSARs) developed were good for all the organisms (R2=0.65 to 0.88; Radj2 = 0.63 to 0.86) and the predictive capability of the developed models was also reasonable (q2=0.52 to 0.83). The models had two to three independent variables. The data for the models which had three independent variables were divided into training and test/validation sets. The former set was used to develop the QSAR and these developed models were used to predict the activity of the test set data. In all the three cases the predictive capability of the models was good. The molecular descriptors identified were predominantly log P, electronic parameters, molecular size, shape and area. A positive correlation existed between the antibacterial activity and the first principal component.已经合成了28种苯并咪唑类衍生物,并对它们对四种细菌(金黄色葡萄球菌、大肠杆菌、短小芽孢杆菌和普通变形杆菌)和两种真菌(黄曲霉和黑曲霉)的抗微生物活性进行了测试。当R为C6H4NO2、R′为SO2C6H4-CH3(p),R为C6H4O 、R′为SO2C6H4- (p),以及R为 C6H5、R′为CH2( )9Cl时,这些化合物的抗菌活性与环丙沙星相当或更高,对金黄色葡萄球菌和大肠杆菌的活性高于制霉菌素对黄曲霉的活性。还有几种化合物对大肠杆菌的活性优于标准抗生素。R为CCl3、R′为SO2C6H4- (p)或COC6H5的化合物对所有生物体的活性最低。在R′位置添加亚甲基基团可以提高活性。许多化合物对一种或多种生物体的活性优于环丙沙星。R为 OC6H5、R′为 ( )9Cl的化合物对这两种真菌的活性高于对照制霉菌素。开发的定量结构-活性关系(QSARs)对所有生物体都很好(R2=0.65至0.88;Radj2 = 0.63至0.86),开发模型的预测能力也合理(q2=0.52至0.83)。这些模型包含两到三个独立变量。具有三个独立变量的模型的数据被分为训练集和测试/验证集。前者用于开发QSAR,这些开发的模型用于预测测试集数据的活性。在所有三种情况下,模型的预测能力都很好。识别的分子描述符主要是log P、电子参数、分子大小、形状和面积。抗菌活性与第一主成分之间存在正相关关系。
-
Studies in Pheromone Biosynthesis: Preparation of<sup>3</sup>H Labelled Precursors of Drosophila Pheromones作者:L. Bricard、G. KuneschDOI:10.1080/00397919308012587日期:1993.9Abstract Two synthetic schemes were designed giving access to tritium labelled potential precursors of Drosophila pheromones. An intermediate in the first scheme allowed the preparation of [3H]-labelled vaccenyl acetate.
-
一种新型乙酰胆碱酯酶抑制剂及其制备方法 和应用
-
MITOCHONDRIA TARGETED CATIONIC ANTI-OXIDANT COMPOUNDS FOR PREVENTION, THERAPY OR TREATMENT OF HYPER-PROLIFERATIVE DISEASE, NEOPLASIAS AND CANCERS申请人:Zarling David A.公开号:US20110059922A1公开(公告)日:2011-03-10The inventions disclosed include methods of treating cancers and related neoplasias, especially prostate cancer, with pharmaceutically acceptable salts comprising lipophilic cation moieties linked to nitroxide or linked to hydroxylamine anti-oxidant groups.
表征谱图
-
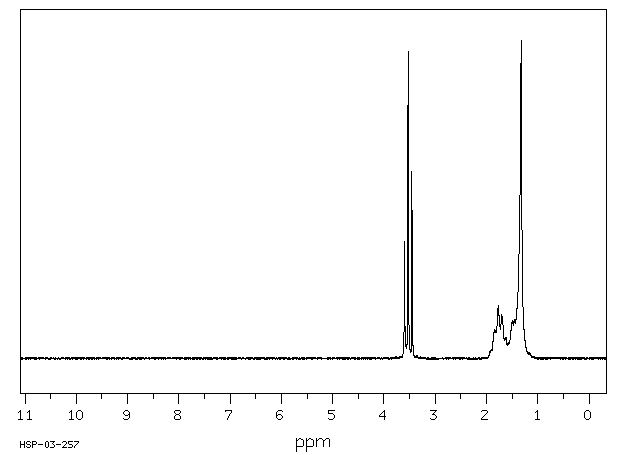
氢谱1HNMR
-
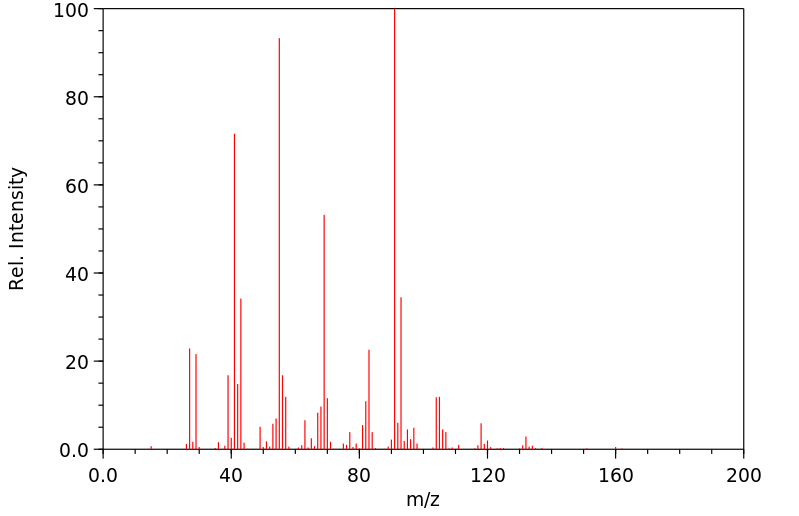
质谱MS
-
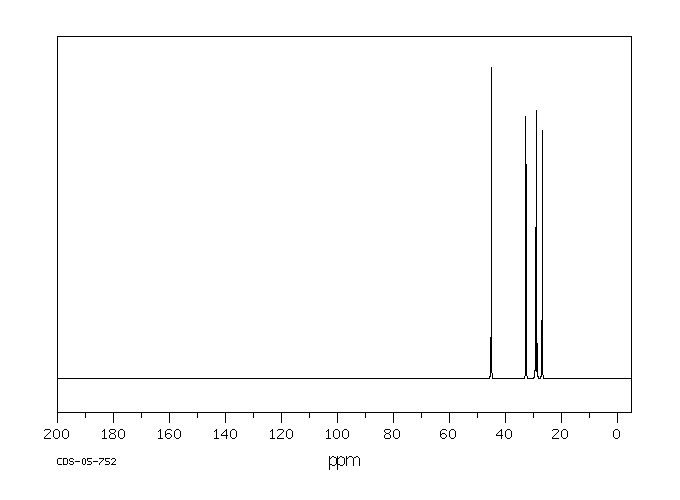
碳谱13CNMR
-
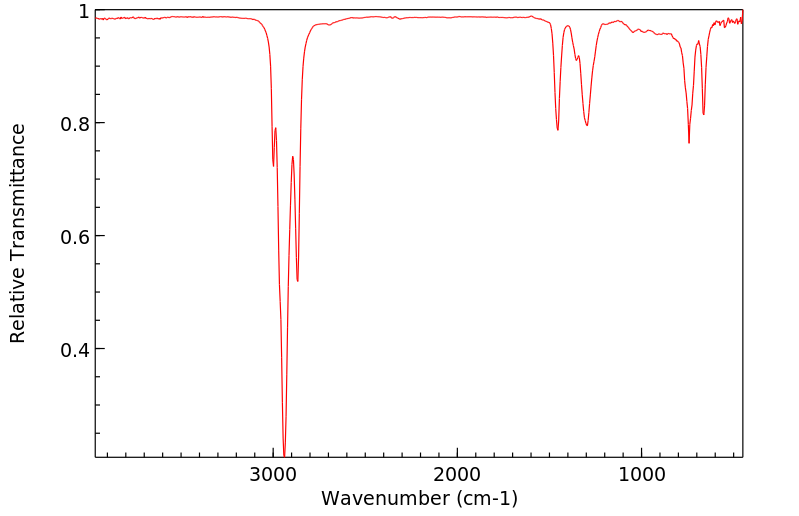
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷