1-(2-氯基-5-磺酸苯基)-3-甲基-5-吡唑酮 | 88-76-6
中文名称
1-(2-氯基-5-磺酸苯基)-3-甲基-5-吡唑酮
中文别名
4-氯-3-(4,5-二氢-3-甲基-5-氧代-1H-吡唑-1-基)-苯磺酸;1-(2-氯-5-磺酸基苯基)-3-甲基-5-吡唑啉酮;3-甲基-1-(2’-氯-5’-磺酸苯基)-5-吡唑啉酮;1-(2'-氯-5'-磺酸苯基)-3-甲基-5-吡唑啉酮;3-甲基-1-(2'-氯-5'-磺酸苯基)-5-吡唑啉酮;2-氯-5-磺酸吡唑酮;1-(2'-氯-5'-磺酸基苯基)-3-甲基-5-吡唑啉酮;25CSMP
英文名称
4-chloro-3-(3-methyl-5-oxo-4,5-dihydropyrazol-1-yl)benzenesulfonic acid
英文别名
4-Chloro-3-(3-methyl-5-oxo-2-pyrazolin-1-yl)benzenesulfonic acid;4-chloro-3-(3-methyl-5-oxo-4H-pyrazol-1-yl)benzenesulfonic acid
CAS
88-76-6
化学式
C10H9ClN2O4S
mdl
MFCD00020745
分子量
288.711
InChiKey
UWLNKHDLVZEYKQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:344 °C(lit.)
-
密度:1?+-.0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:18
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:95.4
-
氢给体数:1
-
氢受体数:5
安全信息
-
危险品标志:Xi
-
危险类别码:R43
-
WGK Germany:3
-
海关编码:2933199090
-
安全说明:S36/37
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H317,H319
制备方法与用途
用途:用于制备染料及颜料的中间体。
反应信息
-
作为反应物:描述:1-(2-氯基-5-磺酸苯基)-3-甲基-5-吡唑酮 、 1,2-萘氧基-二氮杂唑-4-磺酸 在 碳酸氢钠 作用下, 以 乙醇 为溶剂, 以92%的产率得到4-{N'-[1-(2-chloro-5-sulfo-phenyl)-3-methyl-5-oxo-1,5-dihydro-pyrazol-4-ylidene]-hydrazino}-3-hydroxy-naphthalene-1-sulfonic acid参考文献:名称:Hydrazinonaphthalene and Azonaphthalene Thrombopoietin Mimics Are Nonpeptidyl Promoters of Megakaryocytopoiesis摘要:High-throughput screening for the induction of a luciferase reporter gene in a thrombopoietin (TPO)-responsive cell line resulted in the identification of 4-diazo-3-hydroxy-1-naphthalene-sulfonic acids as TPO mimics. Modification of the core structure and adjustment of unwanted functionality resulted in the development of (5-oxo-1,5-dihydropyrazol-4-ylidene)hydrazines which exhibited efficacies equivalent to those of TPO in several cell-based assays designed to measure thrombopoietic activity. Furthermore, these compounds elicited biochemical responses in TPO-receptor-expressing cells similar to those in TPO itself, including kinase activation and protein phosphorylation. Potencies for the best compounds were high for such low molecular weight compounds (MW < 500) with EC50 values in the region of 1-20 nM.DOI:10.1021/jm010283l
文献信息
-
Facile One‐Pot Synthesis and Antimycobacterial Evaluation of Pyrazolo[3,4‐<i>d</i>]pyrimidines作者:Amit Trivedi、Dipti Dodiya、Janak Surani、Samir Jarsania、Hitesh Mathukiya、Naresh Ravat、Viresh ShahDOI:10.1002/ardp.200800027日期:2008.7The present article describes a facile one‐pot synthesis of a series of eight pyrazolo[3,4‐d]pyrimidines 4a–h which were evaluated for their in‐vitro antibacterial activity against Mycobacterium tuberculosis H37Rv using the Alamar‐Blue susceptibility test and the activity expressed as the minimum inhibitory concentration (MIC) in mg/mL. The compounds 4b, 4c, 4d, and 4g exhibited the best results (1
-
Graphene composite, method for producing graphene composite and electrode for lithium ion battery containing graphene composite申请人:TORAY INDUSTRIES, INC.公开号:US10199654B2公开(公告)日:2019-02-05Provided is a graphene composite primarily used as a conductive additive for forming an electrode for lithium ion batteries, which has performance equal to or higher than conventional dispersants and is deceased in cost by using an inexpensive and easily available dispersant. The graphene composite includes a graphene powder and a compound having a structure of pyrazolone.
-
GRAPHENE COMPOSITE, METHOD FOR PRODUCING GRAPHENE COMPOSITE AND ELECTRODE FOR LITHIUM ION BATTERY CONTAINING GRAPHENE COMPOSITE申请人:TORAY INDUSTRIES, INC.公开号:US20160351908A1公开(公告)日:2016-12-01Provided is a graphene composite primarily used as a conductive additive for forming an electrode for lithium ion batteries, which has performance equal to or higher than conventional dispersants and is deceased in cost by using an inexpensive and easily available dispersant. The graphene composite includes a graphene powder and a compound having a structure of pyrazolone.
表征谱图
-
氢谱1HNMR
-
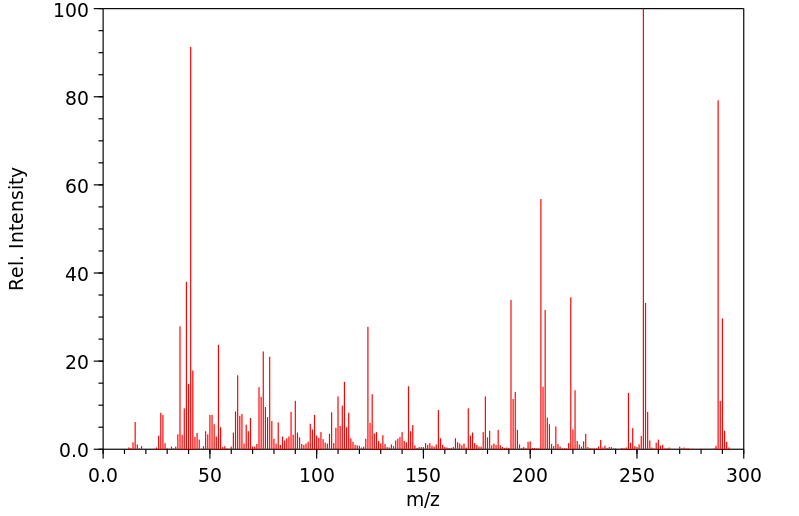
质谱MS
-
碳谱13CNMR
-
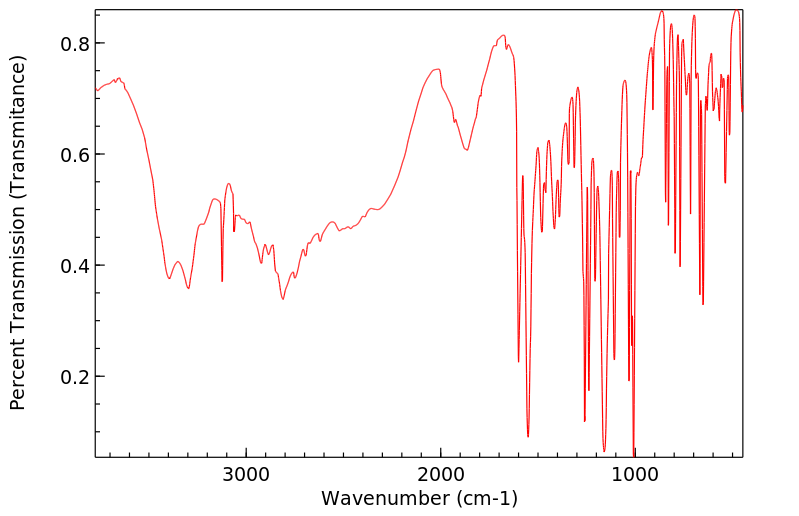
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫