1-(3-氯丙基)-3-苯基脲 | 3420-63-1
中文名称
1-(3-氯丙基)-3-苯基脲
中文别名
——
英文名称
N-(3-Chlor-propyl)-N'-phenyl-harnstoff
英文别名
1-(3-Chloropropyl)-3-phenylurea
CAS
3420-63-1
化学式
C10H13ClN2O
mdl
——
分子量
212.679
InChiKey
YMHWWYTZENGFRG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:41.1
-
氢给体数:2
-
氢受体数:1
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(3-氯丙基)-1-亚硝基-3-苯基脲 1-(3-Chloropropyl)-1-nitroso-3-phenylurea 13907-69-2 C10H12ClN3O2 241.677 1-苯基四氢-2(1h)-嘧啶酮 1-phenyltetrahydropyrimidin-2-one 56535-85-4 C10H12N2O 176.218
反应信息
-
作为反应物:描述:1-(3-氯丙基)-3-苯基脲 在 正丁基锂 、 potassium tert-butylate 作用下, 以 四氢呋喃 、 叔丁醇 为溶剂, 反应 24.0h, 生成 1-Phenyl-3-methyl-3,4,5,6-tetrahydropyrimidin-2(1H)-one参考文献:名称:Cyclic ureas as ortho directing substituents摘要:六成员环脲在通过氮原子与苯环和吡啶环连接时显示出弱的邻位定向能力。DOI:10.1039/b105420c
-
作为产物:描述:参考文献:名称:Braendstroem,A. et al., Acta chemica Scandinavica. Series B: Organic chemistry and biochemistry, 1974, vol. 28, p. 699 - 701摘要:DOI:
文献信息
-
[EN] SUBSTITUTED 2-IMIDAZOLIDONES AND ANALOGS<br/>[FR] 2-IMIDAZOLIDONES SUBSTITUES ET ANALOGUES申请人:UNIV LAVAL公开号:WO2011100840A1公开(公告)日:2011-08-25Compounds of formula (I): wherein R1, R2, R3, R4, R7, R6, R7, R8, R9, A, X and Y as defined herein are provided as useful for the treatment of cancer or for the manufacture of anti-cancer agents.式(I)的化合物:其中所述的R1、R2、R3、R4、R7、R6、R7、R8、R9、A、X和Y如本文所定义的那样,被提供为用于治疗癌症或用于制造抗癌药物。
-
New Substituted 1-(2,3-Dihydrobenzo[1,4]dioxin-2-ylmethyl)piperidin-4-yl Derivatives with α<sub>2</sub>-Adrenoceptor Antagonist Activity作者:Patrice Mayer、Pascale Brunel、Céline Chaplain、Christel Piedecoq、Francis Calmel、Philippe Schambel、Philippe Chopin、Thierry Wurch、Petrus J. Pauwels、Marc Marien、Jean-Louis Vidaluc、Thierry ImbertDOI:10.1021/jm991121g日期:2000.10.1The emergence of a novel theory concerning the role of noradrenaline in the progression and the treatment of neurodegenerative diseases such as Parkinson's and Alzheimer's diseases has provided a new impetus toward the discovery of novel compounds acting at alpha(2)-adrenoceptors. A series of substituted 1-(2,3-dihydrobenzo[1,4]dioxin-2-ylmethyl) piperidin-4-yl derivatives bearing an amide, urea, or imidazolidinone moiety was studied. Some members of this series of compounds proved to be potent alpha(2)-adrenoceptor antagonists with good selectivity versus alpha(1)-adrenergic and D(2)-dopamine receptors. Particular emphasis is given to compound 33g which displays potent alpha(2)-adrenoceptor binding affinity in vitro and central effects in vivo following oral administration.
-
The Synthesis of Potential Anticancer Agents. XXXVI. N-Nitrosoureas.<sup>1</sup> II. Haloalkyl Derivatives作者:Thomas P. Johnston、George S. McCaleb、Pamela S. Opliger、John A. MontgomeryDOI:10.1021/jm00324a026日期:1966.11
-
Design, Synthesis, Biological Evaluation, and Structure–Activity Relationships of Substituted Phenyl 4-(2-Oxoimidazolidin-1-yl)benzenesulfonates as New Tubulin Inhibitors Mimicking Combretastatin A-4作者:Sébastien Fortin、Lianhu Wei、Emmanuel Moreau、Jacques Lacroix、Marie-France Côté、Éric Petitclerc、Lakshmi P. Kotra、René C.-GaudreaultDOI:10.1021/jm200488a日期:2011.7.14Sixty-one phenyl 4-(2-oxoimidazolidin-1-yl)benzenesulfonates (PIB-SOs) and 13 of their tetrahydro-2-oxopyrimidin-1(2H)-yl analogues (PPB-SOs) were prepared and biologically evaluated. The antiproliferative activities of PIB-SOs on 16 cancer cell lines are in the nanomolar range and unaffected in cancer cells resistant to colchicine, paclitaxel, and vinblastine or overexpressing the P-glycoprotein. None of the PPB-SOs exhibit significant antiproliferative activity. PIB-SOs block the cell cycle progression in the G(2)/M phase and bind to the colchicine-binding site on beta-tubulin leading to cytoskeleton disruption and cell death. Chick chorioallantoic membrane tumor assays show that compounds 36, 44, and 45 efficiently block angiogenesis and tumor growth at least at similar levels as combretastatin A-4 (CA-4) and exhibit low to very low toxicity on the chick embryos. PIB-SOs were subjected to CoMFA and CoMSIA analyses to establish quantitative structure-activity relationships.
-
Siefken, Justus Liebigs Annalen der Chemie, 1949, vol. 562, p. 75,1114作者:SiefkenDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
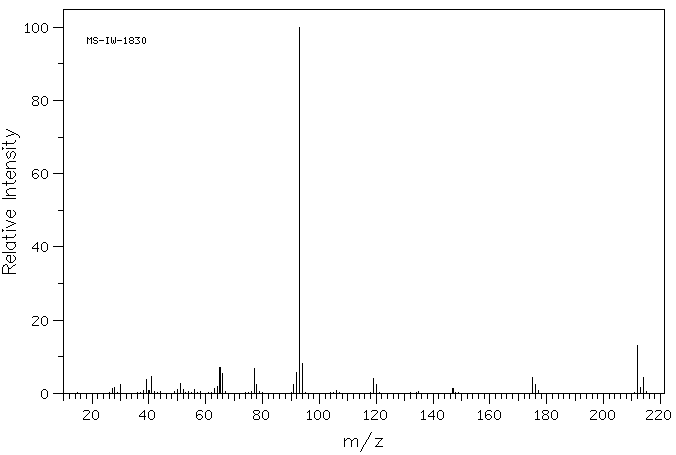
质谱MS
-
碳谱13CNMR
-
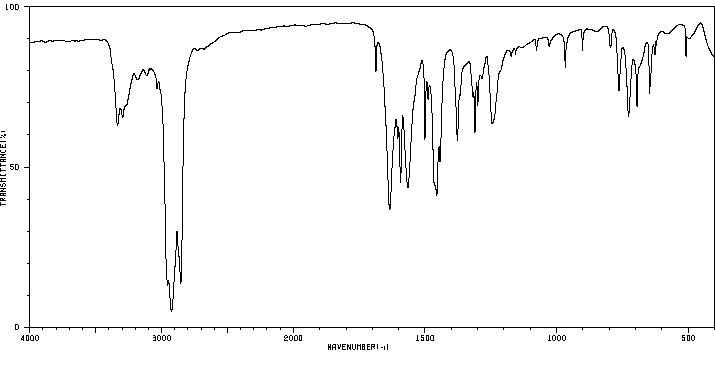
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫