棕榈酸对硝基苯酯 | 1492-30-4
中文名称
棕榈酸对硝基苯酯
中文别名
4-硝基苯基棕榈酸酯;棕榈酸4-硝基苯酯;对硝基苯酚棕榈酸酯;十六碳酸4-硝基苯酯;4-硝基苯棕榈酸酯;4-硝基苯
英文名称
4-nitrophenyl palmitate
英文别名
p-nitrophenyl palmitate;pNPP;para-nitrophenyl palmitate;p-nitrophenol palmitate;p-NP palmitate;palmitic acid-para-nitrophenylester;(4-nitrophenyl) hexadecanoate
CAS
1492-30-4
化学式
C22H35NO4
mdl
MFCD00047732
分子量
377.524
InChiKey
LVZSQWIWCANHPF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:65-66°C
-
沸点:483.6±28.0 °C(Predicted)
-
密度:1.020±0.06 g/cm3(Predicted)
-
溶解度:二甲基甲酰胺:1mg/mL
-
稳定性/保质期:
如果按照规格使用和储存,就不会分解,没有已知的危险反应。应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):8.8
-
重原子数:27
-
可旋转键数:16
-
环数:1.0
-
sp3杂化的碳原子比例:0.681
-
拓扑面积:72.1
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S36/37
-
危险类别码:R43
-
WGK Germany:3
-
海关编码:2915709000
-
危险标志:GHS07
-
危险性描述:H317
-
危险性防范说明:P280
-
储存条件:密封,在-20°C下保存
SDS
1.1 产品标识符
: 4-Nitrophenyl palmitate
产品名称
1.2 鉴别的其他方法
HexadECanoic acid 4-nitrophenyl ester
p-Nitrophenyl palmitate
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤敏化作用 (类别1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H317 可能导致皮肤过敏反应。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P272 禁止将受污染的工作服带出工作场地.
P280 戴防护手套。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P321 具体治疗(见本标签上提供的急救指导)。
P333 + P313 如发生皮肤刺激或皮疹:求医/ 就诊。
P363 沾染的衣服清洗后方可重新使用。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: HexadECanoic acid 4-nitrophenyl ester
别名
p-Nitrophenyl palmitate
: C22H35NO4
分子式
: 377.52 g/mol
分子量
组分 浓度或浓度范围
4-Nitrophenyl palmitate
-
CAS 号 1492-30-4
EC-编号 216-084-9
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
建议的贮存温度: -20 °C
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 60 °C
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
会引起过敏性皮肤反应。
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 4-Nitrophenyl palmitate
产品名称
1.2 鉴别的其他方法
HexadECanoic acid 4-nitrophenyl ester
p-Nitrophenyl palmitate
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤敏化作用 (类别1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H317 可能导致皮肤过敏反应。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P272 禁止将受污染的工作服带出工作场地.
P280 戴防护手套。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P321 具体治疗(见本标签上提供的急救指导)。
P333 + P313 如发生皮肤刺激或皮疹:求医/ 就诊。
P363 沾染的衣服清洗后方可重新使用。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: HexadECanoic acid 4-nitrophenyl ester
别名
p-Nitrophenyl palmitate
: C22H35NO4
分子式
: 377.52 g/mol
分子量
组分 浓度或浓度范围
4-Nitrophenyl palmitate
-
CAS 号 1492-30-4
EC-编号 216-084-9
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
建议的贮存温度: -20 °C
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 60 °C
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
会引起过敏性皮肤反应。
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
用途:酯酶底物。
上下游信息
反应信息
-
作为反应物:参考文献:名称:Characterization, performance, and applications of a yeast surface display-based biocatalyst摘要:酵母表面展示(YSD)两种脂肪酶。测量表达水平和拷贝数。合成和水解活性与商业脂肪酶相当。YSD系统与商业配方的成本分析。DOI:10.1039/c4ra16304d
-
作为产物:参考文献:名称:对-[N,N-双(2-氯乙基)氨基]苯酚的一系列脂肪酸酯的合成和生物评价。摘要:合成了一系列对-[N,N-双(2-氯乙基)氨基]苯酚的偶数脂肪酸酯(C2-C18),并评估了其对L-1210小鼠白血病的急性毒性和有效性。通过癸酸酯衍生物的乙酸盐在HA / ICR小鼠中显示出的毒性是苯酚芥子碱的2至3倍。月桂酸酯,肉豆蔻酸酯,棕榈酸酯和硬脂酸酯衍生物的溶解度较低。对于所有化合物,除乙酸盐和己酸酯衍生物外,在白血病研究中均观察到显着的存活时间(T / C%大于或等于125)。肉豆蔻酸衍生物的存活时间增长最大,达到162%。棕榈酸酯和硬脂酸酯衍生物分别在五个和四个剂量水平下产生了显着的存活率。DOI:10.1002/jps.2600710713
文献信息
-
Alkyl carbamate-substituted beta-lactones, process and intermediate products for their preparation, and pharmaceutical compositions containing them申请人:Antel Jochen公开号:US20050197386A1公开(公告)日:2005-09-08Substituted β-lactones (oxetanones) corresponding to the formula I, wherein R 1 , R 2 and n have the meanings given in the specification, and pharmaceutical compositions which contain these compounds and have a pancreatic lipase-inhibiting action, as well as a process for the preparation of the compounds of Formula I and intermediate products of this process.
-
Photoaffinity-labeled sphingomyelin analogs and processes thereof申请人:Katsumura Shigeo公开号:US20050182265A1公开(公告)日:2005-08-18A photoaffinity-labeled sphingomyelin analog of the following formula: wherein R 1 is C 1-20 alkylene group, R 5 is C 1-20 alkyl group, aryl group or C 1-6 alkyl group substituted by aryl group, Z is photoaffinity-labeled group and Me is methyl group, or an optically active compound thereof.以下公式的光亲和标记鞘磷脂类似物: 其中R1是C1-20烷基基团,R5是C1-20烷基基团、芳基基团或由芳基基团取代的C1-6烷基基团,Z是光亲和标记基团,而Me是甲基基团, 或者是其光学活性化合物。
-
Salt Effects on Solvolysis Reactions of <i>p</i>-Nitrophenyl Alkanoates Catalyzed by 4-(Dialkylamino)pyridine- Functionalized Polymer in Buffered Water and Aqueous Methanol Solutions作者:Guang-Jia Wang、Donghao Ye、Wilmer K. FifeDOI:10.1021/ja961505l日期:1996.1.1salting-in agent, from the Tris buffer system. The salting-in effect on formation of 1·2 complex is presumed responsible for the substrate specificity. The salting-out effects of sodium chloride on the solvolysis of 2 catalyzed by 1 were also investigated in 1:1 (v/v) methanol−water solution at pH 8.0 and 30 °C. The rate of 1-catalyzed sol...对于由 4-(二烷基氨基)吡啶官能化聚合物 1 在 pH 8.0 的水性 Tris 缓冲溶液中催化的对硝基苯基链烷酸酯 2(n = 2-16)的水解,观察到导致显着底物选择性的特定盐析效应和30℃。发现大分子 1 在 0.05 M Tris 缓冲水溶液中对 2 (n = 6) 表现出明显的底物偏好,这与在 0.05 M 磷酸盐或硼酸盐缓冲溶液中不存在底物选择性的相应反应形成对比。Tris 缓冲系统中三(羟甲基)甲基铵离子(一种有效的盐渍剂)的存在似乎促进了反应性催化剂-底物复合物 1·2 的形成。1·2 复合物形成的盐渍效应被认为是底物特异性的原因。还在 pH 8.0 和 30 °C 的 1:1 (v/v) 甲醇-水溶液中研究了氯化钠对 2 由 1 催化的溶剂分解的盐析效应。1-催化溶胶率...
-
Sphingolipids and Glycerolipids. IV. Syntheses and Ionophoretic Activities of Several Analogues of Soya-cerebroside II, a Calcium Ionophoretic Sphingoglycolipid Isolated from Soybean.作者:Hirotaka SHIBUYA、Michio KUROSU、Kazuyuki MINAGAWA、Seiji KATAYAMA、Isao KITAGAWADOI:10.1248/cpb.41.1534日期:——For examination of the structure-activity relationship five analogues [(2'R)-2'-hydroxypalmitoyl (3), palmitoyl (4), (2'S)-2'-hydroxypalmitoyl (5), β-D-galactosyl (6), and 8, 9-dihydro (7) relevancies] of soya-cerebroside II (2), which is a calcium ionophoretic sphingoglycolipid isolated from soybean, have been synthesized by using our previously reported synthetic method for sphingoglycolipids. Examinations by using a W-08 (liquid-membrane type) apparatus and by means of the human erythrocyte membrane method, have shown that the (2'R)-2'-hydroxypalmitoyl analogue (3) exhibits higher ion-binding and ion-permeation activities for calcium ion than soya-cerebroside II (2), which contains 3 as the major component. It has also been found that the other analogues(5, 6, 7, 8) do not show those ionophoretic activities.An enantiomer (8) of the (2'R)-2'-hydroxypalmitoyl analogue (3) has been synthesized and its calcium ionophoretic activity examined. Compound 8 exhibits calcium ion-binding activity equal to that of 3, but 8 lacks the ability to support calcium ion-permeation through human erythrocyte membrane. Thus, human erythrocyte membrane precisely distinguishes the absolute configurations of 3 and 8 as regards calcium ionophoretic activity.为了研究结构-活性关系,我们利用先前报道的鞘糖脂合成方法,合成了大豆神经酰胺II(2)的五种类似物[(2'R)-2'-羟基棕榈酰(3)、棕榈酰(4)、(2'S)-2'-羟基棕榈酰(5)、β-D-半乳糖基(6)和8, 9-二氢(7)相关物],2是从大豆中分离出的钙离子载体鞘氨醇糖脂。通过使用W-08(液膜型)装置和人体红细胞膜方法的检测表明,(2'R)-2'-羟基棕榈酰类似物(3)对钙离子的离子结合和离子渗透活性高于以3为主要成分的大豆神经酰胺II(2)。还发现其他类似物(5, 6, 7, 8)没有那些离子载体活性。合成了(2'R)-2'-羟基棕榈酰类似物(3)的对映体(8),并检测了其钙离子载体活性。化合物8显示出与3相等的钙离子结合活性,但8缺乏支持钙离子通过人体红细胞膜的能力。因此,人体红细胞膜在钙离子载体活性方面精确区分了3和8的绝对构型。
-
Selective Decarbonylation of Fatty Acid Esters to Linear α-Olefins作者:Alex John、Büsra Dereli、Manuel A. Ortuño、Hillis E. Johnson、Marc A. Hillmyer、Christopher J. Cramer、William B. TolmanDOI:10.1021/acs.organomet.7b00411日期:2017.8.14mixed-ligand system exploiting the trans-spanning diphosphine XantPhos and an N-heterocyclic carbene (IPr) was identified as the most effective in yielding high α-selectivity and high conversions of the ester (>98% selectivity, >90% conversion using 2.5 mol % of PdCl2 and 5 mol % of the ligands, 190 °C, 2–2.5 h). On the basis of insights from modeling at the density functional level of theory, we propose
表征谱图
-
氢谱1HNMR
-
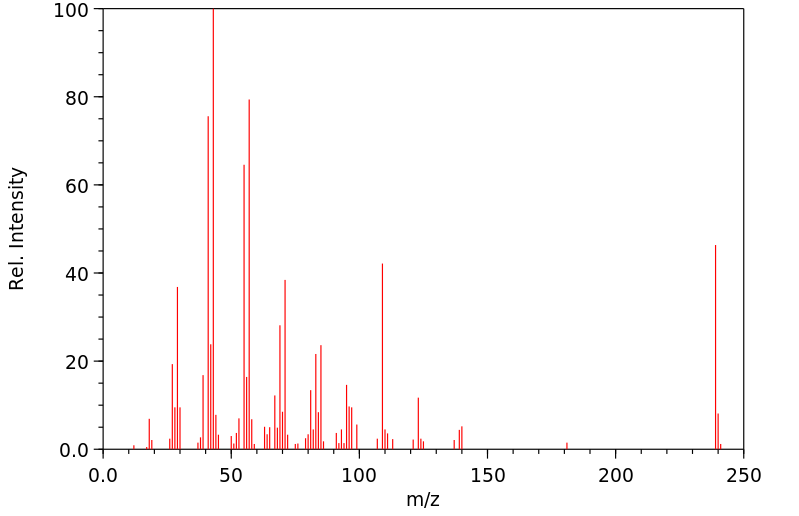
质谱MS
-
碳谱13CNMR
-
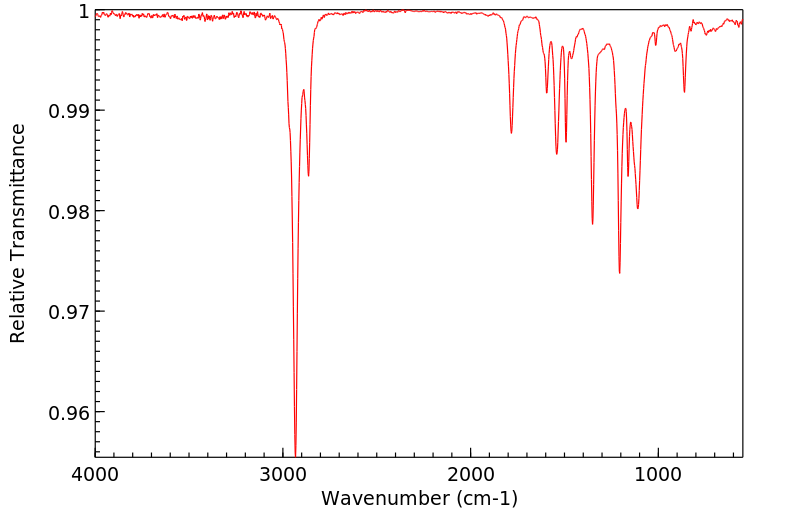
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰亚胺四聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺六聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺-酰胺-PEG8-四氟苯酚酯
马来酰亚胺-四聚乙二醇-五氟苯酯
马来酰亚胺-三聚乙二醇-五氟苯酚酯
靛酚乙酸酯
阿立哌唑标准品002
间硝基苯基戊酸酯
间氯苯乙酸乙酯
间乙酰苯甲酸
钾4-乙酰氧基苯磺酸酯
酚醛乙酸酯
邻苯二酚二乙酸酯
邻甲苯基环己甲酸酯
邻甲氧基苯乙酸酯
辛酸苯酯
辛酸对甲苯酚酯
辛酸五氯苯基酯
辛酸-(3-氯-苯基酯)
辛酰溴苯腈
苯酰胺,3,4-二(乙酰氧基)-N-[6-氨基-1,2,3,4-四氢-1-(4-甲氧苯基)-3-甲基-2,4-二羰基-5-嘧啶基]-
苯酚-乳酸
苯酚,4-异氰基-,乙酸酯(ester)
苯酚,4-[(四氢-2H-吡喃-2-基)氧代]-,乙酸酯
苯酚,3-(1,1-二甲基乙基)-,乙酸酯
苯酚,2-溴-3-(二溴甲基)-5-甲氧基-,乙酸酯
苯甲醇,4-(乙酰氧基)-3,5-二甲氧基-
苯甲酸,4-(乙酰氧基)-2-氟-
苯氧基氯乙酸苯酯
苯基金刚烷-1-羧酸酯
苯基氰基甲酸酯
苯基庚酸酯
苯基庚-6-炔酸酯
苯基己酸酯
苯基呋喃-2-羧酸酯
苯基吡啶-2-羧酸酯
苯基十一碳-10-烯酸酯
苯基乙醛酸酯
苯基乙酸酯-d5
苯基丙二酸单苯酯
苯基丙-2-炔酸酯
苯基丁-2,3-二烯酸酯
苯基4-乙基环己烷羧酸
苯基3-乙氧基-3-亚氨基丙酸盐
苯基2-(苯磺酰基)乙酸酯
苯基2-(4-甲氧基苯基)乙酸酯
苯基2-(2-甲氧基苯基)乙酸酯
苯基2-(2-甲基苯基)乙酸酯
苯基-乙酸-(2-甲酰基-苯基酯)
苯基-乙酸-(2-环己基-苯基酯)