正-己基三甲氧基硅烷 | 3069-19-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:<0°C
-
沸点:202 °C
-
密度:0,92 g/cm3
-
闪点:62°C
-
溶解度:不溶于水
-
LogP:0.1-3.2 at 20℃
-
物理描述:Liquid
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:13
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:27.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:Yes
-
安全说明:S26,S36/37/39
-
危险类别码:R36/38
-
海关编码:2931900090
-
危险品标志:Xn
-
危险品运输编号:1993
-
危险性防范说明:P210,P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313,P370+P378,P403+P235,P501
-
危险性描述:H227,H315,H319
-
储存条件:存放于0-6°C阴凉干燥处
SDS
Section I.Chemical Product and Company Identification
Chemical Name n-Hexyltrimethoxysilane
Portland OR
Synonym Not available.
CH3(CH2)5Si(OCH3)3
Chemical Formula
CAS Number 3069-19-0
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
n-Hexyltrimethoxysilane 3069-19-0 Min. 96.0 Not available. Not available.
(GC)
Section III. Hazards Identification
Acute Health Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is
not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. DO NOT use an eye ointment. Flush eyes with running water for a minimum
of 15 minutes, occasionally lifting the upper and lower eyelids. Seek medical attention. Treat symptomatically and
supportively.
Skin Contact If the chemical gets spilled on a clothed portion of the body, remove the contaminated clothes as quickly as possible,
protecting your own hands and body. Place the victim under a deluge shower. If the chemical touches the victim's
exposed skin, such as the hands: Gently and thoroughly wash the contaminated skin with running water and non-abrasive
soap. Be particularly careful to clean folds, crevices, creases and groin. Cover the irritated skin with an emollient. Seek
medical attention. Treat symptomatically and supportively. Wash any contaminated clothing before reusing.
Inhalation If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or waistband. If
breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt, or waistband. If the victim is not breathing, administer artificial respiration.
Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material
was ingested; the absence of such signs, however, is not conclusive. Seek immediate medical attention and, if possible,
show the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Not available.
Flammability Combustible. Auto-Ignition
Flash Points Flammable Limits
77°C (170.6°F). Not available.
Combustion Products
These products are toxic carbon oxides (CO, CO 2). Some metallic oxides.
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Continued on Next Page
3069- n-Hexyltrimethoxysilane
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO 2, water spray or foam.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Section VI. Accidental Release Measures
Spill Cleanup Combustible material.
Instructions Keep away from heat and sources of ignition. Mechanical exhaust required. Stop leak if without risk. Finish cleaning the
spill by rinsing any contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities
for assistance on disposal.
Section VII. Handling and Storage
Handling and Storage COMBUSTIBLE. Keep away from heat and sources of ignition. Mechanical exhaust required. When not in use, tightly
seal the container and store in a dry, cool place. Avoid excessive heat and light. Do not breathe gas, fumes, vapor or
Information
spray. In case of insufficient ventilation, wear suitable respiratory equipment. If you feel unwell, seek medical attention
and show the label when possible. Treat symptomatically and supportively. Avoid contact with skin and eyes.
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Personal Protection Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. A MSHA/NIOSH approved respirator must be used to avoid
inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling
this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Liquid. Not available.
0.92
Specific Gravity
Molecular Weight 206.36 Partition Coefficient Not available.
Boiling Point
Not available. Vapor Pressure Not available.
Melting Point Not available. Vapor Density Not available.
Refractive Index Not available. Volatility Not available.
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
Not available.
RTECS Number
Eye contact. Inhalation. Ingestion.
Routes of Exposure
Toxicity Data Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
Continued on Next Page
n-Hexyltrimethoxysilane
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local or regional authorities. You may be able to dissolve or mix material with
Waste Disposal
a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state, and local regulations when disposing of this substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This compound is ON the EPA Toxic Substances Control Act (TSCA) inventory list.
(EPA)
WHMIS Classification
WHMIS CLASS B-3: Combustible liquid with a flash point between 37.8°C (100°F) and 93.3°C (200°F).
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
正己基三甲氧基硅烷是一种透明至稻黄色的液体,带有温和气味。作为一种典型的硅烷偶联剂,分子中的己烷使其表现出优异的理化性能,并具有广泛的应用场景。
这种物质可应用于尼龙、PBT等工程塑料的加纤、填充等改性处理中,有助于提高改性塑料的机械强度;它还能用于对硅微粉进行表面处理,进而增强环氧树脂模塑料、环氧树脂砂浆及环氧树脂模具的性能。此外,在建筑和玻璃用聚硫密封胶中替代巯基硅烷使用时,正己基三甲氧基硅烷具有良好的增粘效果,并且能避免巯基硅烷带来的不舒适气味。另外,它还能加入到聚氨酯和胶乳类胶粘剂中,显著提高其粘接力,不会导致产品变黄或影响储存期,与氨基硅烷偶联剂相比表现更优。
正己基三甲氧基硅烷的制备方法如下:以三甲氧基硅烷(TMSH)和己烯为原料,通过硅氢加成反应合成该化合物。在氯铂酸配合物中添加两种助催化剂(氮化合物和磷化合物),以此来调整加成反应的选择性和反应速度。
制备步骤-
原料处理:先用分子筛将各原料进行脱水干燥处理24小时,称取69.2g(0.20 mol)己烯、0.692 g催化剂(铂含量为5000 ppm,与烯烃的质量比为1:20000),以及29.8 g(0.22 mol)三甲氧基硅烷。确保催化剂与反应物完全混合均匀,并保持干燥状态,防止与水接触。
-
设备准备:在高压釜内进行干燥处理并抽空,利用负压将反应物料吸入后密闭。
-
加热反应:加热升温至120℃,维持2小时。反应过程中会放热,请控制温差不超过20℃。随后将温度提高至140℃,再保持2小时,直至反应结束降温至室温后出料进行精馏提纯,并通过气相分析检测产物正己基三甲氧基硅烷的质量。
另一种制备方法的详细试剂和仪器信息如下:
所用主要设备包括:集热式恒温加热磁力搅拌器(型号DF -101S)及气相色谱仪(型号GC-14D,由上海精密科学仪器有限公司生产)、气-质联用仪(型号HP -6890GC/5973MS,由HP公司制造)。
反应信息
-
作为反应物:描述:正-己基三甲氧基硅烷 在 bis(1,5-cyclooctadiene)nickel (0) 、 C34H18F10IrN4(1+)*F6P(1-) 、 potassium methanolate 、 4,4'-二叔丁基-2,2'-二吡啶 作用下, 以 甲醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 27.0h, 生成 4-正己基苯甲腈参考文献:名称:硅酸盐作为潜在的烷基自由基前体:高价双邻苯二酚硅化合物的可见光光催化氧化摘要:这项工作介绍了高价双邻苯二酚硅化合物作为可见光光催化时烷基自由基的通用来源。使用Ir [(dF(CF 3)ppy)2(bpy)](PF 6)(dF(CF 3)ppy = 2-(2,4-二氟苯基)-5-三氟甲基吡啶,bpy = bipyridine)作为催化光氧化剂,可以产生一系列的烷基,包括高反应性的伯基,并参与各种分子间的均解反应。在循环伏安法,斯特恩-沃尔默研究的基础上,并在计算的支持下,提出了一种机制,该机制涉及从硅酸盐到光活化铱配合物的单电子转移。这种氧化光催化过程可以有效地与镍催化的C merge合并C交叉偶联反应。DOI:10.1002/anie.201504963
-
作为产物:描述:参考文献:名称:Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Si: MVol.C, 74, page 207 - 208摘要:DOI:
文献信息
-
Photoredox‐Catalyzed Cyclopropanation of 1,1‐Disubstituted Alkenes via Radical‐Polar Crossover Process作者:Wenping Luo、Yi Yang、Yewen Fang、Xinxin Zhang、Xiaoping Jin、Guicai Zhao、Li Zhang、Yan Li、Wanli Zhou、Tingting Xia、Bin ChenDOI:10.1002/adsc.201900708日期:2019.9.171‐disubstituted alkenes via radical addition‐anionic cyclization cascade has been successfully developed. Another new protocol based on photocatalytic allylation and cyclopropanation cascade was also described between allylic halide and halomethyl radical. In addition to the successful use of bis‐catecholato silicates as the alkyl radical precursors, the acyl and alkyl radicals derived from 1,4‐dihydropyridines已成功开发了通过自由基加成-阴离子环化级联反应对1,1-二取代烯烃进行光氧化还原中性催化的环丙烷化反应。还描述了另一种基于光催化烯丙基化和环丙烷化级联的新方案,介于烯丙基卤化物和卤代甲基之间。除了成功地使用双邻苯二酚硅酸盐作为烷基自由基前体外,从1,4-二氢吡啶衍生的酰基和烷基也参与了该自由基-极性交叉过程。竞争实验显示的3-外- TET环化的模式优于4-外型和5-外型环化模式,从而允许选择性3-外型- TET环化。在溴甲基自由基与均烯丙基(假)卤化物的反应中,已经证明了溴化物比氯化物和甲苯磺酸盐优越的核沉着特征。该新协议的特点是其氧化还原中性过程,广泛的底物范围,温和的条件以及良好的功能基团相容性。
-
一种以可循环使用的铂化合物为催化剂的硅 氢加成反应
-
一种硅氢加成反应的方法
-
Synthesis of hydrosilanes <i>via</i> Lewis-base-catalysed reduction of alkoxy silanes with NaBH<sub>4</sub>作者:Keiya Aoyagi、Yu Ohmori、Koya Inomata、Kazuhiro Matsumoto、Shigeru Shimada、Kazuhiko Sato、Yumiko NakajimaDOI:10.1039/c9cc01961h日期:——Hydrosilanes were synthesized by reduction of alkoxy silanes with BH3 in the presence of hexamethylphosphoric triamide (HMPA) as a Lewis-base catalyst. The reaction was also achieved using an inexpensive and easily handled hydride source NaBH4, which reacted with EtBr as a sacrificial reagent to form BH3in situ.
-
ボラン還元を用いたヒドロシランの製造方法申请人:国立研究開発法人産業技術総合研究所公开号:JP2018100231A公开(公告)日:2018-06-28【課題】ヒドロシランを効率良く製造することができるヒドロシランの製造方法を提供することを目的とする。【解決手段】ルイス塩基の存在下で下記式(a)で表される構造を有するシランとボラン錯体又はジボランを反応させることにより、ヒドロシランが効率よく製造することができる。(式(a)中、R1は炭素原子数1〜20の炭化水素基、又は炭素原子数1〜10のアシル基を表す。)【選択図】なし
表征谱图
-
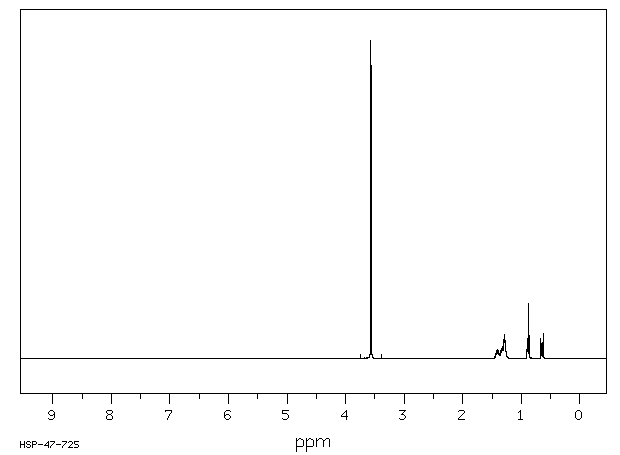
氢谱1HNMR
-
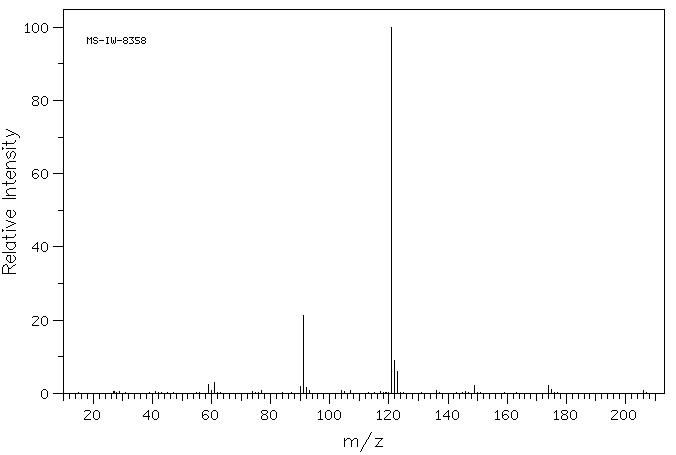
质谱MS
-
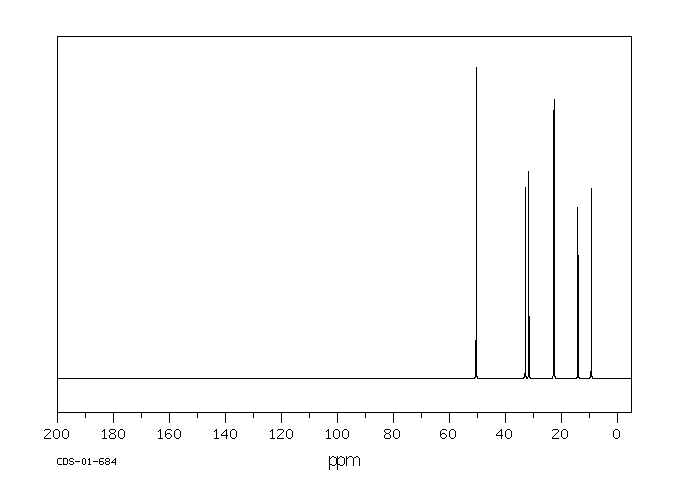
碳谱13CNMR
-
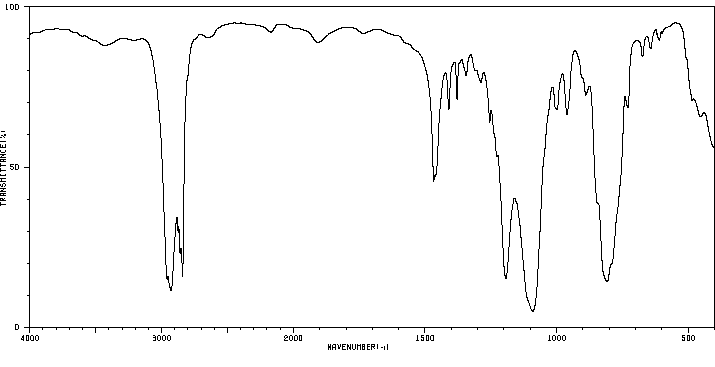
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息