1-氟壬烷 | 463-18-3
中文名称
1-氟壬烷
中文别名
——
英文名称
1-fluorononane
英文别名
1-Fluor-nonan
CAS
463-18-3
化学式
C9H19F
mdl
——
分子量
146.248
InChiKey
ITPAUTYYXIENLO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-40°C
-
沸点:166-169 °C
-
密度:0.81
-
闪点:48 °C
-
保留指数:957
-
稳定性/保质期:
避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):5.3
-
重原子数:10
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:3.2
-
危险品标志:Xn,Xi
-
安全说明:S16,S26,S36/37/39,S37/39,S45
-
危险类别码:R20/21/22,R10,R36/37/38
-
RTECS号:RA6605000
-
包装等级:III
-
危险类别:3.2
-
危险品运输编号:1993
-
储存条件:密封储存于阴凉、干燥的库房,远离热源、火花和火焰。
SDS
| Name: | 1-Fluorononane 98% Material Safety Data Sheet |
| Synonym: | Nonyl Fluoride |
| CAS: | 463-18-3 |
Synonym:Nonyl Fluoride
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 463-18-3 | 1-Fluorononane | 98% | unlisted |
Risk Phrases: 10 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Vapors may cause dizziness or suffocation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Water may be ineffective. Material is lighter than water and a fire may be spread by the use of water. Containers may explode in the heat of a fire.
Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Wash clothing before reuse. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 463-18-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 166 - 169 deg C @ 760.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 48 deg C ( 118.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .8160g/cm3
Molecular Formula: C9H19F
Molecular Weight: 146.25
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 463-18-3: RA6605000 LD50/LC50:
CAS# 463-18-3: Oral, rat: LD50 = 3 mg/kg.
Carcinogenicity:
1-Fluorononane - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 10 Flammable.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 33 Take precautionary measures against static
discharges.
S 37 Wear suitable gloves.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 463-18-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 463-18-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 463-18-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1,9-difluorononane 223919-32-2 C9H18F2 164.239
反应信息
-
作为反应物:描述:参考文献:名称:氟代烷烃的还原脱氟摘要:在亚化学计量的1,2-双(三甲基甲硅烷基)苯1的存在下,过量的锂粉和催化量的DTBB与伯,仲和叔氟烷烃反应,得到相应的烷烃,这是由氟-氢交换产生的。该方法可以扩展到非二元的二氟化物。还研究了二甲硅烷基化的化合物在萘催化的氟苯和苄基氟化物的锂化反应中的作用。DOI:10.1016/s0040-4020(03)00019-x
-
作为产物:参考文献:名称:Kawana, Masajiro; Nishikawa, Masahiro; Yamasaki, Noritsugu, Journal of the Chemical Society. Perkin transactions I, 1989, p. 1593 - 1596摘要:DOI:
文献信息
-
Synthesis of alkyl halides under neutral conditions作者:François Munyemana、Anne-Marie Frisque-Hesbain、Alain Devos、Léon GhosezDOI:10.1016/s0040-4039(00)99407-3日期:1989.1Primary and secondary alcohols are efficiently converted to the corresponding alkyl halides under neutral conditions.伯醇和仲醇在中性条件下可有效地转化为相应的烷基卤。
-
Copper-Catalyzed Regioselective Hydroalkylation of 1,3-Dienes with Alkyl Fluorides and Grignard Reagents作者:Takanori Iwasaki、Ryohei Shimizu、Reiko Imanishi、Hitoshi Kuniyasu、Nobuaki KambeDOI:10.1002/anie.201503288日期:2015.8.3Copper complexes generated in situ from CuCl2, alkyl Grignard reagents, and 1,3‐dienes play important roles as catalytic active species for the 1,2‐hydroalkylation of 1,3‐dienes by alkyl fluorides through CF bond cleavage. The alkyl group is introduced to an internal carbon atom of the 1,3‐diene regioselectively, thus giving rise to the branched terminal alkene product.
-
Reactions of 1-fluoroalkyl triflates with nucleophiles and bases作者:William R. Dolbier、Masamune OkamotoDOI:10.1016/j.jfluchem.2015.01.013日期:2015.11A series of 1-fluoroalkyl triflates are prepared, isolated and characterized. Their reactions with a large variety of nucleophiles are described. From these reactions are obtained 1-fluoroalkyl nitriles, azides, formates, acetates, ethers, phenylthio ethers, triphenylphosphonium salts, benztriazoles, benzimidazoles, xanthates, iodides, bromides and chlorides, most in excellent yield.
-
Cross-coupling Reaction of Alkyl Halides with Alkyl Grignard Reagents Catalyzed by Cp-Iron Complexes in the Presence of 1,3-Butadiene作者:Takanori Iwasaki、Ryohei Shimizu、Reiko Imanishi、Hitoshi Kuniyasu、Nobuaki KambeDOI:10.1246/cl.180201日期:2018.6.5cross-coupling reaction of alkyl halides with alkyl Grignard reagents by the combined use of cyclopentadienyl ligand and 1,3-butadiene additive is described. The reaction smoothly proceeds at room temperature using unactivated alkyl bromides and fluorides via non-radical mechanism, which is in sharp contrast with hitherto known Fe-catalyzed cross-coupling reactions of alkyl halides.
-
Process for preparing sil acyclohexane-based liquid crystal compounds from silacyclohexanone compounds申请人:SHIN-ETSU CHEMICAL CO., LTD.公开号:EP0765880A1公开(公告)日:1997-04-02Silacyclohexane-based liquid crystal compounds are prepared from a cyclohexanone compound of the following general formula (I) wherein Ar represents a phenyl group or a tolyl group, and R represents a phenyl group, a tolyl group, a linear alkyl group having from 1 to 10 carbon atoms, a mono or difluoroalkyl group having from 1 to 10 carbon atoms, a branched alkyl group having from 3 to 8 carbon atoms or an alkoxyalkyl group having from 2 to 7 carbon atoms, and also from organolithium reagents, followed by hydrogenolysis or hydrogenation after dehydration, with or without further reaction with organometal reagents, and then to de-silylation and reduction.
表征谱图
-
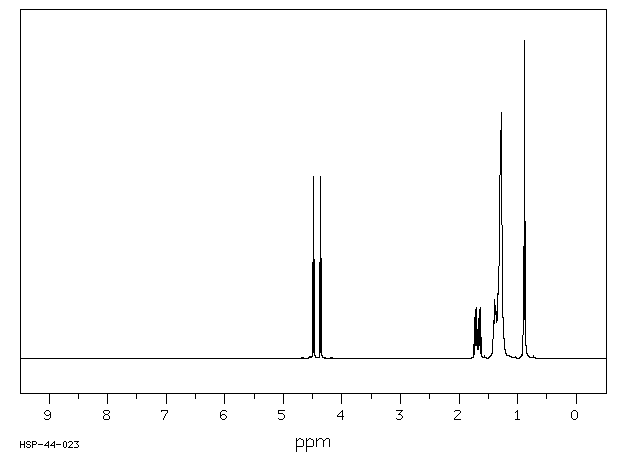
氢谱1HNMR
-
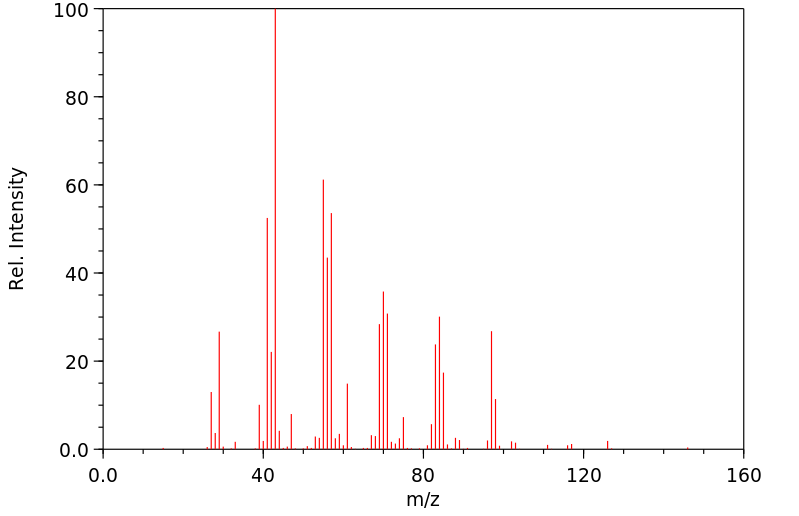
质谱MS
-
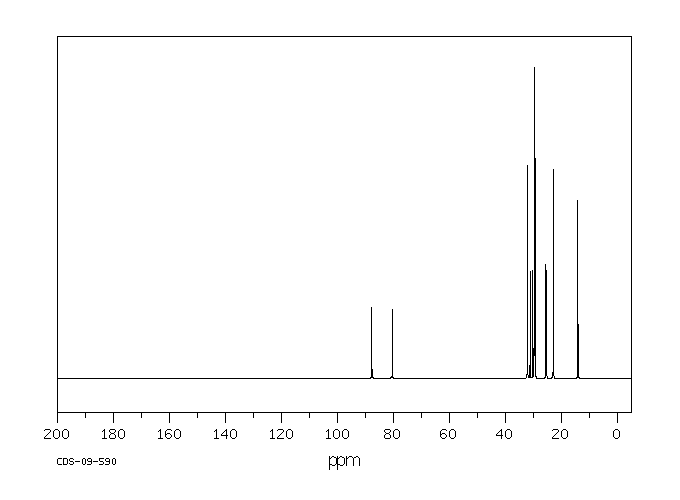
碳谱13CNMR
-
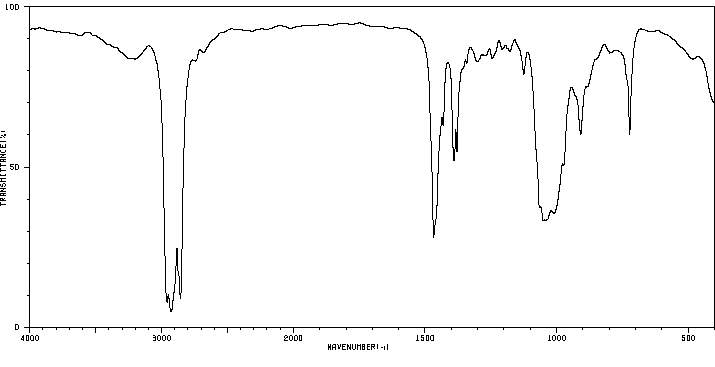
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-氟-环丙胺
顺式-1,1,1,4,4,4-六氟-2-丁烯
顺-1,1,2,2,3,4-六氟环丁烷
酰亚胺基二亚磷酸,甲基-,四(2,2,2-三氟乙基)酯
舒巴坦酸
聚(7-脱氮杂腺嘌呤酸)
癸烷,6-溴-1,1,1,2,2,3,3-七氟-4,4-二(三氟甲基)-
环丙基溴化镁
溴五氟乙烷
氯氟烃-252
氯氟烃-232
氯氟-甲基
氯四氟乙烷
氯二氟乙醛
氯三氟乙烷
氨甲酸,(氟磺酰)-,甲基酯
氢氯氟碳-261
氟甲醇
氟甲基自由基
氟甲基环戊烷
氟甲基环丙烷
氟环辛烷
氟环戊烷
氟环庚烷
氟环十二烷
氟环丁烷
1-溴-1-氯-2,2,2-三氟乙烷
氟氯乙烷
氟化烯丙基
氟化乙亚胺酰基,2-(二氟氨基)-N,2,2-三氟-
氟化丁基
氟乙醛
氟乙烷
氟乙烯醚
正膦胺,N-(2,3,4,5,6-五氯-2,3,4,5,6-五氟亚环己基)-1,1,1,1-四(2,2,3,3-四氟丙氧基)-
桉叶素
替氟烷
恩氟烷
异氟醚
异十八烷酸己酯
己酸,2,5-二氨基-6-羟基-(7CI)
奥替尼啶HCL
壬氟环戊烷
地氟烷
叔丁基氟化物
反式-2-氟环丙胺盐酸盐
反式-2-氟代环戊烷-1-胺盐酸盐
反式-1,2-双(全氟己基)乙烯
反式-1,2-双(全氟-n-丁基)乙烯
反式-1,1,1,2,2,3,3-七氟-4-壬烯