1-甲基-5-硝基-1H-吲唑 | 5228-49-9
中文名称
1-甲基-5-硝基-1H-吲唑
中文别名
——
英文名称
1-methyl-5-nitro-1H-indazole
英文别名
1-Methyl-5-nitroindazol;1-methyl-5-nitroindazole
CAS
5228-49-9
化学式
C8H7N3O2
mdl
MFCD01318163
分子量
177.162
InChiKey
JHPMRMBDPINHAV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:161-162°C
-
沸点:332.9±15.0 °C(Predicted)
-
密度:1.42±0.1 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:13
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:63.6
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R20/22
-
危险品运输编号:2811
-
海关编码:2933990090
-
包装等级:II
-
安全说明:S22,S36/37
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:常温下应存放在避光、阴凉且干燥的地方,并密封保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1-Methyl-5-nitro-1h-indazole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-Methyl-5-nitro-1h-indazole
CAS number: 5228-49-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H7N3O2
Molecular weight: 177.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1-Methyl-5-nitro-1h-indazole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-Methyl-5-nitro-1h-indazole
CAS number: 5228-49-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H7N3O2
Molecular weight: 177.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-硝基吲唑 5-nitroindazole 5401-94-5 C7H5N3O2 163.136 1-甲基-5-硝基-1H-吲唑-3-胺 1-methyl-5-nitro-1H-indazol-3-ylamine 73105-48-3 C8H8N4O2 192.177 6-硝基吲唑 6-nitroindazole 7597-18-4 C7H5N3O2 163.136 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-氨基-1-甲基-1H-吲唑 1-methyl-1H-indazol-5-amine 50593-24-3 C8H9N3 147.18 1-甲基-5-硝基-1H-吲唑-3-胺 1-methyl-5-nitro-1H-indazol-3-ylamine 73105-48-3 C8H8N4O2 192.177 3-溴-1-甲基-5-硝基-1H-咪唑 3-bromo-1-methyl-5-nitro-1H-indazole 74209-25-9 C8H6BrN3O2 256.059 —— 3-chloro-1-methyl-5-nitro-1H-indazole 74209-24-8 C8H6ClN3O2 211.608 —— Methyl-(1-methyl-5-nitro-1H-indazol-3-yl)-amine 77894-75-8 C9H10N4O2 206.204 —— N,N,1-trimethyl-5-nitro-1H-indazol-3-amine 77894-76-9 C10H12N4O2 220.231 —— 1-methyl-5-nitro-3-phenyl-1H-indazole 728035-42-5 C14H11N3O2 253.26 1-甲基-1H-吲唑-5-醇 1-methyl-1H-indazol-5-ol 756839-14-2 C8H8N2O 148.164 —— 2-[(1-Methylindazol-5-yl)amino]benzoic acid 132283-39-7 C15H13N3O2 267.287
反应信息
-
作为反应物:描述:1-甲基-5-硝基-1H-吲唑 在 钯 氢气 、 铜 作用下, 以 乙醇 、 戊醇 为溶剂, 反应 8.0h, 生成 2-[(1-Methylindazol-5-yl)amino]benzoic acid参考文献:名称:Boyer, Gerard; Galy, Jean-Pierre; Faure, Robert, Journal of Chemical Research, Miniprint, 1990, # 11, p. 2601 - 2615摘要:DOI:
-
作为产物:描述:参考文献:名称:新的硝基吲哚基乙腈:通过取代的亲核取代和由阴离子介导的互变异构转换而实现的有效合成途径†摘要:通过用4-氯苯氧基乙腈将N-甲基-硝基吲唑类化合物进行亲核取代,可以有效地获得新的N-烷基-硝基吲哚基乙腈。所有化合物均已通过NMR和质谱技术进行了充分表征,其中一些化合物的结构还通过X射线衍射分析数据得到了证实。加入碱性阴离子后,在该系列硝基吲哚基乙腈中观察到互变异构转换,然后进行UV-Vis分光光度法和1 H-NMR滴定。阴离子官能团诱导的互变异构物质的形成得到密度泛函理论计算的支持。DOI:10.1039/c9nj02807b
文献信息
-
[EN] CONDENSED HETEROCYCLIC COMPOUNDS HAVING 5-HT6 RECEPTOR AFFINITY<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES CONDENSÉS AYANT UNE AFFINITÉ POUR LE RÉCEPTEUR 5-HT6申请人:MEMORY PHARM CORP公开号:WO2010021797A1公开(公告)日:2010-02-25The present disclosure provides compounds having affinity for the 5-HT6 receptor which are of the formula (I) wherein R1, A, B, D, E, G, Q, Ar, n, m, and p are as defined herein. The disclosure also relates to methods of preparing such compounds, compositions containing such compounds, and methods of use thereof.本公开提供了具有亲和力的化合物,其对5-HT6受体具有亲和力,其化学式为(I),其中R1、A、B、D、E、G、Q、Ar、n、m和p如本文所定义。该公开还涉及制备这种化合物的方法、含有这种化合物的组合物以及使用方法。
-
[EN] 4'-AMINO CYCLIC COMPOUNDS HAVING 5-HT6 RECEPTOR AFFINITY<br/>[FR] COMPOSÉS 4'-AMINO CYCLIQUES PRÉSENTANT UNE AFFINITÉ POUR LE RÉCEPTEUR 5-HT6申请人:MEMORY PHARM CORP公开号:WO2010024980A1公开(公告)日:2010-03-04The present disclosure provides compounds having affinity for the 5-HT6 receptor which are of the formula (I): formula (I) wherein Cy is selected from formula (Il) and wherein R1, R2, R3, Q, G, Ar, m, n and p are as defined herein. The disclosure also relates to methods of preparing such compounds, compositions containing such compounds, and methods of use thereof.
-
New heterocyclic green, blue and orange dyes from indazole: Synthesis, tautomerism, alkylation studies, spectroscopic characterization and DFT/TD-DFT calculations作者:Soodabeh Poorhaji、Mehdi Pordel、Shirin RamezaniDOI:10.1016/j.molstruc.2016.04.078日期:2016.9Abstract Tautomerism and alkylation studies on the green intermediate 2-(5-hydroxyimino-1-methyl-4,5-dihydro-1 H -4-indazolyliden)-2-phenylacetonitrile led to the synthesis of new heterocyclic green, blue and orange dyes in high yields. The structures of all newly synthesized compounds were confirmed by spectral and analytical data. The optical properties of the dyes were spectrally characterized by
-
[EN] N-ALKYNYL-2- (SUBSTITUTED ARYLOXY) ALKYLTHIOAMIDE DERIVATIVES AS FUNGICIDES<br/>[FR] DERIVES DE N-ALKYNYLE-2- (SUBSTITUE ARYLOXY) ALKYLTHIOAMIDE COMME FONGICIDES申请人:SYNGENTA LTD公开号:WO2004108663A1公开(公告)日:2004-12-16Fungicidal compounds of the general formula (1), wherein Ar is a group of the formula (A), (B1), (B2) or (C), or Ar is a 5- or 6-linked group of the formula (D1) or (D2); and R1, R2, R3, R4, R5, n, A1, A2, A3, A4, A5, Ka, Kb, L, M, V, W, X,Y and Z have the definitions given in claim 1.一般公式(1)中的杀菌化合物,其中Ar是公式(A)、(B1)、(B2)或(C)的组,或Ar是5-或6-连接的公式(D1)或(D2)的组;且R1、R2、R3、R4、R5、n、A1、A2、A3、A4、A5、Ka、Kb、L、M、V、W、X、Y和Z具有权利要求1中给出的定义。
-
Nucleophilic and Radical Heptafluoroisopropoxylation with Redox‐Active Reagents作者:Chao‐Lai Tong、Xiu‐Hua Xu、Feng‐Ling QingDOI:10.1002/anie.202109572日期:2021.10.11heptafluoroisopropoxylation reactions through the invention of a series of redox-active N-OCF(CF3)2 reagents. These reagents were readily prepared from the oxidative heptafluoroisopropylation of hydroxylamines with AgCF(CF3)2. The substitutions on the nitrogen atom significantly affected the properties and reactivities of N-OCF(CF3)2 reagents. Accordingly, two types of N-OCF(CF3)2 reagents including N-OCF(CF3)2七氟异丙基 (CF(CF 3 ) 2 ) 在药物和农用化学品中很普遍。然而,七氟异丙氧基化(OCF(CF 3 ) 2 ) 化合物在很大程度上仍未得到充分探索,这可能是由于缺乏对这些化合物的有效获取。在此,我们通过一系列具有氧化还原活性的N -OCF(CF 3 ) 2试剂的发明,公开了实用且高效的七氟异丙氧基化反应。这些试剂很容易通过羟胺与 AgCF(CF 3 ) 2的氧化七氟异丙基化反应制备. 氮原子上的取代显着影响了N -OCF(CF 3 ) 2试剂的性质和反应性。因此,两种类型的N -OCF(CF 3 ) 2试剂包括N -OCF(CF 3 ) 2邻苯二甲酰亚胺A和N -OCF(CF 3 ) 2苯并三唑鎓盐O'用作OCF(CF 3 ) 2分别为阴离子和自由基前体。该协议能够对一系列底物进行直接七氟异丙氧基化,以中等至优异的产量提供相应的产品。
表征谱图
-
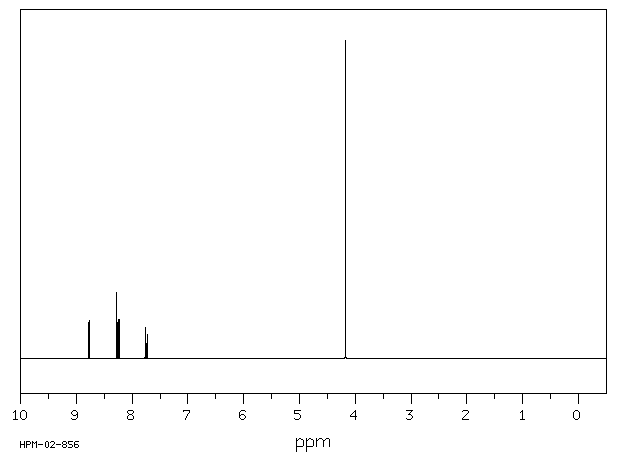
氢谱1HNMR
-
质谱MS
-
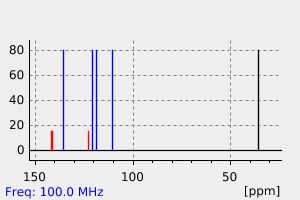
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙基N-(1H-吲唑-3-基羰基)ethanehydrazonoate)
(2R,6S)-2,6-二甲基-4-[6-[5-(1-(甲基环丙基)氧基]-1H-吲唑-3-基]嘧啶-4-基]吗啉
顺式-八氢-2-甲基-吡咯并[3,4-c]吡咯
陀尼达安
阿西替尼杂质
阿布查米卡代谢物M4
达泽达明
苯达扎克钠
苯甲酸,5-(溴甲基)-2-硝基-,甲基酯
苯乙胺杂质8
苯丙酰胺,b-氨基-3-乙氧基-
苄达酸钠盐
苄达酸
苄达明 N-氧化物
苄达明
苄胺N-氧化物马来酸氢盐
苄基-(3-氯-5-硝基-1H-吲唑-7-基)-胺
盐酸苄达明
1-(2,4-二氯苄基)-1H-吲唑-3-碳酰肼
甲基7-氨基-1H-吲唑-3-羧酸酯
甲基5-溴-1-(四氢-2H-吡喃-2-基)-1H-吲唑-3-甲酸基酯
甲基4-碘-1H-吲唑-6-羧酸
甲基4-氨基-1-乙基-1H-吲唑-6-羧酸酯
甲基-6-硝基-1H-吲唑-3-羧酸
甲基 1H-吲唑-7-羧酸盐酸盐
水杨酸化合物与3-[(1-苄基-1H-吲唑-3-基)氧基]-N,N-二甲基丙基胺(1:1)
氯尼达明
格拉斯琼杂质C
格拉司琼相关物质C
格拉司琼相关物质A
格拉司琼氮氧化物
帕唑帕尼相关化合物2
帕唑帕尼杂质57
希尼达明
宾达利
奈米利塞
奈拉米诺
因旦尼定
四溴甲基-1-甲基吲唑
哌啶,2-乙基-1-(3-苯基-2-丙炔-1-基)-
吲熟酯
吲唑-6-硼酸
吲唑-5-甲酸盐酸盐
吲唑-5-甲酸甲酯
吲唑-5-甲腈
吲唑-4-硼酸盐酸盐
吲唑-4-乙酸
吲唑-3-羧酸乙脂
吲唑-3-羧酸
吲唑-3-乙酸