10-羟基-10-苯基蒽-9-酮 | 5146-30-5
中文名称
10-羟基-10-苯基蒽-9-酮
中文别名
——
英文名称
10-phenyl-10-hydroxy-9-anthracenone
英文别名
10-Hydroxy-10-phenyl-anthron;9-Hydroxy-9-phenyl-anthron;10-Hydroxy-10-phenyl-9-anthron;9-hydroxy-9-phenylanthrone;10-Hydroxy-10-phenyl-anthron-(9);10-Hydroxy-10-phenyl-9(10H)-anthracenone;10-hydroxy-10-phenylanthracen-9-one
CAS
5146-30-5
化学式
C20H14O2
mdl
——
分子量
286.33
InChiKey
GPYIJEJINDRVKK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:22
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:0.05
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2914400090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— n-Butyltritylonether 73476-13-8 C24H22O2 342.437 —— n-Decyltritylonether 73476-14-9 C30H34O2 426.599 —— n-Octyltritylonether 36112-48-8 C28H30O2 398.545 —— 5-Tritylonoxy-2-pentanol 73476-15-0 C25H24O3 372.464 —— 10-[4-(4-Cyanbenzyloxy)pentyloxy]-10-phenyl-9(10H)-anthracenon 73476-16-1 C33H29NO3 487.598 —— 10-phenylanthrone 14596-70-4 C20H14O 270.331 —— 9,10-Epoxy-9,10-dihydro-10-phenylanthracene 142230-36-2 C20H14O 270.331 9,10-二苯基蒽内过氧化物 9,10-diphenyl-9,10-epidioxyanthracene 15257-17-7 C26H18O2 362.428 —— 2-(diphenylmethyl)benzoic acid 602-50-6 C20H16O2 288.346 —— 10-chloro-10-phenyl-anthrone 52236-50-7 C20H13ClO 304.776 蒽醌 9,10-phenanthrenequinone 84-65-1 C14H8O2 208.216 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 10-Methoxy-10-phenyl-anthron 25548-89-4 C21H16O2 300.357 —— n-Butyltritylonether 73476-13-8 C24H22O2 342.437 —— n-Octyltritylonether 36112-48-8 C28H30O2 398.545 —— n-Decyltritylonether 73476-14-9 C30H34O2 426.599 —— 5-Tritylonoxy-2-pentanol 73476-15-0 C25H24O3 372.464 —— 4-Chlor-1-(tritylonoxy)pentan 77588-19-3 C25H23ClO2 390.909 —— 10-[4-(4-Cyanbenzyloxy)pentyloxy]-10-phenyl-9(10H)-anthracenon 73476-16-1 C33H29NO3 487.598 9,10-二氢-9,10-二苯基蒽-9,10-二醇 anthranol 6318-17-8 C26H20O2 364.444 —— 10-phenylanthrone 14596-70-4 C20H14O 270.331 10,10-二苯基-蒽酮 10,10-Diphenyl-10H-anthracen-9-one 3216-03-3 C26H18O 346.428 10-(4-羟基苯基)-10-苯基蒽-9-酮 10-(4-hydroxy-phenyl)-10-phenyl-anthrone 7508-39-6 C26H18O2 362.428 —— 10-(4-methoxy-phenyl)-10-phenyl-anthrone 6941-82-8 C27H20O2 376.455 —— 10-chloro-10-phenyl-anthrone 52236-50-7 C20H13ClO 304.776 蒽醌 9,10-phenanthrenequinone 84-65-1 C14H8O2 208.216 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Hydroxy- and Methoxyphenylanthrones. III1摘要:DOI:10.1021/ja01868a084
-
作为产物:描述:2-(diphenylmethyl)benzoic acid 在 sodium dichromate 、 溶剂黄146 、 zinc(II) chloride 作用下, 生成 10-羟基-10-苯基蒽-9-酮参考文献:名称:Hydroxy- and Methoxyphenylanthrones. III1摘要:DOI:10.1021/ja01868a084
文献信息
-
Reactions of cupric halides with organic compounds—VII作者:A.D. Mosnaim、D.C. Nonhebel、J.A. RussellDOI:10.1016/s0040-4020(01)98790-3日期:1970.19-Alkoxy(or acyloxy)-10-methylanthracenes undergo reaction with cupric halides at the Me group to give coupled products, whereas 9-alkoxy(or acyloxy)-10-benzyl(or ethyl)anthracenes react at the alkoxy or acyloxy group to afford 10-benzyl- and 10-ethylideneanthrones respectively. Both types of reaction are postulated to proceed by a radical mechanism.
-
Reactions of copper(II) halides with aromatic compounds—XI作者:J.M. Mancilla、D.C. Nonhebel、J.A. RussellDOI:10.1016/0040-4020(75)80155-4日期:1975.19-Alkyl and 9-arylanthracenes react with copper(II) bromide in methanol to give 10-alkyl (or aryl)-10-methoxyanthrones as the major product. 9-Methylanthracene additionally affords 10-methoxy-10-methoxymethylanthrone. The formation of all these products can be interpreted in terms of an initial electron-transfer oxidation of the aromatic moiety to the radical cation.
-
Substituent Effects on the Ionization Equilibria of 9-Aryl-9-fluorenols and 10-Aryl-10-hydroxyanthracen-9-ones作者:Trevor W. Toone、Edward Lee-Ruff、Alan C. HopkinsonDOI:10.1139/v75-230日期:1975.6.1
The title compounds were prepared and their basicities measured. In both systems the major part of the molecule is held planar, while an aryl group is free to rotate. This may interact with the positive charge but steric hindrance to the ortho hydrogens may be expected to oppose planarity. In these systems no evidence was found for a reduction of the normal σ+ value due to failure to achieve planarity. A formula is presented which gives the approximate degree of resemblance (α) of the transition state to the products and, in the case of the ionization of triarylmethanols, α is found to be linear with pKR+. It is suggested that this could resolve the apparent contradiction between the Hammett equation and the Hammond postulate.
这些标题化合物已经制备并测量了它们的碱性。在这两个体系中,分子的主要部分保持平面,而芳基团可以自由旋转。这可能会与正电荷发生相互作用,但对邻位氢的立体位阻可能会阻碍平面性。在这些体系中,没有发现由于无法实现平面性而导致正常σ+值降低的证据。提出了一个公式,该公式给出了过渡态与产物之间近似相似度(α),在三芳基甲醇离子化的情况下,发现α与pKR+呈线性关系。建议这可能解决汉密特方程与哈蒙德假设之间的明显矛盾。 -
Photo-oxygénation d'amines aromatiques endoperoxydes d'amino-9 anthracènes作者:Jean Santamaria、Jean RigaudyDOI:10.1016/0040-4020(80)80223-7日期:1980.1The direct photo-oxygenation of 10-phenyl and 10-methoxy-9-dimethylaminoanthracenes 1a and 1b gives the corresponding 9,10-endoperoxides 2a and 2b. The latter are unstable owing to their high reactivity towards nucleophiles.
-
The oxidation of organic compounds by “singlet” oxygen作者:E. McKeown、William A. WatersDOI:10.1039/j29660001040日期:——The normal or “triplet” ground state (3Σg–) of molecular oxygen is that of a biradical, ·O–O·, but “singlet” oxygen (1Δg) OO, which is structurally similar to ethylene, is the initial product of heterolytic decompositions of hydrogen peroxide or of per-acids. “Singlet” oxygen reverts to normal “triplet” oxygen by a bimolecular reaction that produces chemiluminescence but is sufficiently long-lived
表征谱图
-
氢谱1HNMR
-
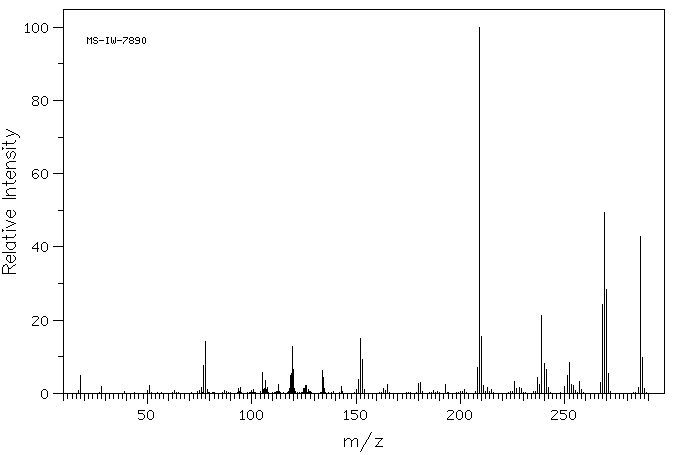
质谱MS
-
碳谱13CNMR
-
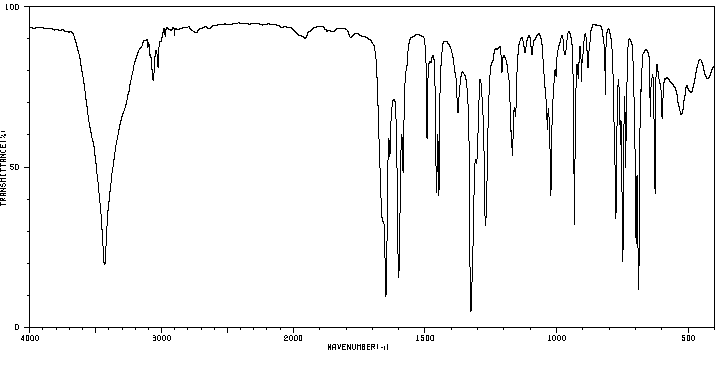
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62