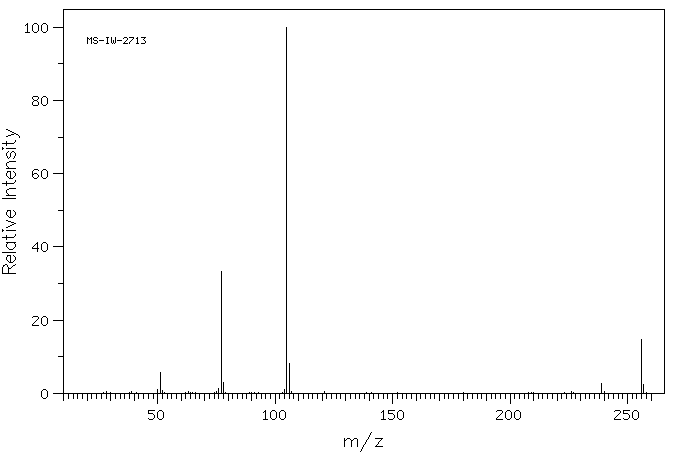
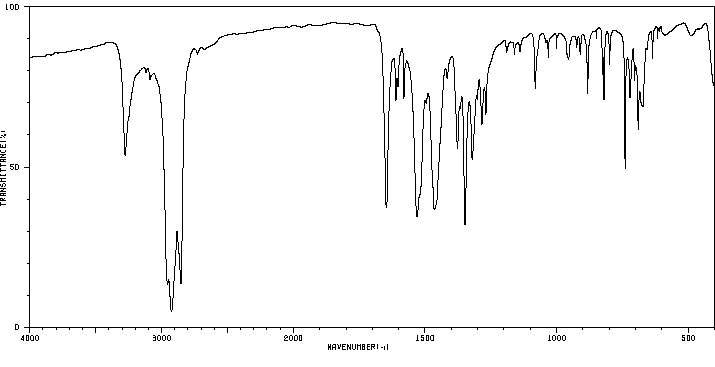
2'-甲基-5'-硝基苯甲酰苯胺 | 4771-07-7
中文名称
2'-甲基-5'-硝基苯甲酰苯胺
中文别名
——
英文名称
N-(2-methyl-5-nitrophenyl)benzamide
英文别名
——
CAS
4771-07-7
化学式
C14H12N2O3
mdl
MFCD00430481
分子量
256.261
InChiKey
AITINBWGCGZTNE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:330.1±35.0 °C(Predicted)
-
密度:1.304±0.06 g/cm3(Predicted)
-
溶解度:8.5 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:19
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:74.9
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(2-甲基-5-氨基苯基)苯甲酰胺 N-(2-methyl-5-aminophenyl)benzamide 223138-39-4 C14H14N2O 226.278 —— N-(3-benzamido-4-methylphenyl)-3,5-bis(trifluoromethyl)benzamide 1374006-57-1 C23H16F6N2O2 466.383 —— N-[5-[(3,4-dimethylphenyl)carbamoylamino]-2-methylphenyl]benzamide 1374006-46-8 C23H23N3O2 373.455 —— N-[5-[(3,5-dichlorophenyl)carbamoylamino]-2-methylphenyl]benzamide 1374006-42-4 C21H17Cl2N3O2 414.291 —— N-[5-[(2-chlorophenyl)carbamoylamino]-2-methylphenyl]benzamide 1374006-40-2 C21H18ClN3O2 379.846 —— N-[5-[(3,4-dichlorophenyl)carbamoylamino]-2-methylphenyl]benzamide 1374006-41-3 C21H17Cl2N3O2 414.291 —— N-[5-[(3-acetylphenyl)carbamoylamino]-2-methylphenyl]benzamide 1374006-47-9 C23H21N3O3 387.438 —— N-[2-methyl-5-[(3-phenoxyphenyl)carbamoylamino]phenyl]benzamide 1374006-48-0 C27H23N3O3 437.498 —— N-(3-benzamido-4-methylphenyl)-4-chloro-3-(trifluoromethyl)benzamide 1374006-59-3 C22H16ClF3N2O2 432.829 —— N-[5-[[3,5-bis(trifluoromethyl)phenyl]carbamoylamino]-2-methylphenyl]benzamide 1374006-43-5 C23H17F6N3O2 481.397 —— N-(3-benzamido-4-methylphenyl)-2,3-dihydro-1,4-benzodioxine-6-carboxamide 328959-39-3 C23H20N2O4 388.423 —— N-(3-benzamido-4-methylphenyl)-5-methyl-1,2-oxazole-3-carboxamide 1374006-60-6 C19H17N3O3 335.362 —— N-[5-[(6-chloropyridin-3-yl)carbamoylamino]-2-methylphenyl]benzamide 1374006-51-5 C20H17ClN4O2 380.834 —— N-[5-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-2-methylphenyl]benzamide 1374006-44-6 C22H17ClF3N3O2 447.844 —— N-[5-(1,3-benzodioxol-5-ylcarbamoylamino)-2-methylphenyl]benzamide 1374006-49-1 C22H19N3O4 389.411 —— N-[5-(2,3-dihydro-1,4-benzodioxin-6-ylcarbamoylamino)-2-methylphenyl]benzamide 1374006-50-4 C23H21N3O4 403.437 —— N-[5-[[3-fluoro-5-(trifluoromethyl)phenyl]carbamoylamino]-2-methylphenyl]benzamide 1374006-45-7 C22H17F4N3O2 431.389 - 1
- 2
反应信息
-
作为反应物:描述:2'-甲基-5'-硝基苯甲酰苯胺 在 palladium on activated charcoal 、 氢气 、 1-羟基苯并三唑 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 三乙胺 作用下, 以 甲醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 14.0h, 生成 N-(3-benzamido-4-methylphenyl)-2,3-dihydro-1,4-benzodioxine-6-carboxamide参考文献:名称:New diarylureas and diarylamides possessing acet(benz)amidophenyl scaffold: Design, synthesis, and antiproliferative activity against melanoma cell line摘要:A series of new diarylurea and diarylamide derivatives possessing acet(benz)amidophenyl scaffold was synthesized. Their in vitro antiproliferative activity was tested against A375P human melanoma cell line. Compounds 1c,d and 2c,d showed the highest potencies with IC50 values in sub-micromolar scale. In addition, compounds 1b,e,l and 2e,l were more potent than Sorafenib but with IC50 values in micromolar range. Moreover, compound 2c was equipotent to Vemurafenib, and 2d showed higher potency than Vemurafenib against A375P. Molar refractometry calculation and ADME profiling of the highest potent four derivatives 1c,d and 2c,d are also reported. (C) 2012 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2012.03.020
-
作为产物:描述:参考文献:名称:N-(2-甲基-5-硝基-苯基)苯甲酰胺有机单晶的合成、光谱分析和DFT研究摘要:N-(2-甲基-5-硝基-苯基)苯甲酰胺(N2M5NPBA)化合物是通过2-甲基-5-硝基苯胺的苯甲酰化合成的。N2M5NPBA 单晶是通过缓慢蒸发技术使用 DMSO 作为溶剂生长的。N2M5NPBA晶体的单晶X射线衍射研究在空间群P 2 1/C下具有单斜晶系,晶格参数a = 4.9490 Å, b = 10.1916 Å, c = 24.969 Å, α = γ = 90°, β = 91 ° 和 V = 1259.1 Å 3. N2M5NPBA 晶体中存在的硝基和酰胺官能团在 FT-IR 光谱表征中得到鉴定。优化了标题分子的分子几何结构,并使用 B3LYP/6-311++G (d, p) 基组通过密度泛函理论 (DFT) 进行了理论分析。N2M5NPBA 的紫外-可见光谱分析预测截止波长为 278 nm(带隙能量 ~4.19 eV)。1小时 & 13C NMR谱记录生长的N2M5NPBA晶体以确认化学结构。生长的DOI:10.1016/j.molstruc.2021.131172
文献信息
-
Amide derivatives申请人:Brown S. Dearg公开号:US20050245551A1公开(公告)日:2005-11-03The invention concerns amide derivatives of Formula (Ia) wherein X is —NHCO— or —CONH—; m is 0-3; R 1 is a group such as hydroxy, halogeno, trifluoromethyl, cyano, mercapto, nitro, amino, carboxy and carbamoyl; n is 0-2; R 2 is a group such as hydroxy, halogeno, trifluoromethyl, cyano, mercapto, nitro, amino and carboxy; R 3 is hydrogen, halogeno, (1-6C)alkyl or (1-6C)alkoxy; q is 0-4; and Q is a group such as aryl, aryloxy, aryl-(1-6C)alkoxy, arylamino and N -(1-6C)alkyl-arylamino; or pharmaceutically-acceptable salts or in-vivo-cleavable esters thereof; processes for their preparation, pharmaceutical compositions containing them and their use in the treatment of diseases or medical conditions mediated by cytokines.
-
Bourhim, Mustapha; Poupaert, Jacques H.; Stables, James P., Arzneimittel-Forschung/Drug Research, 1999, vol. 49, # 2, p. 81 - 87作者:Bourhim, Mustapha、Poupaert, Jacques H.、Stables, James P.、Vallee, Louis、Vamecq, JosephDOI:——日期:——
-
Acylthiourea, Acylurea, and Acylguanidine Derivatives with Potent Hedgehog Inhibiting Activity作者:Antonio Solinas、Hélène Faure、Hermine Roudaut、Elisabeth Traiffort、Angèle Schoenfelder、André Mann、Fabrizio Manetti、Maurizio Taddei、Martial RuatDOI:10.1021/jm2013369日期:2012.2.23The Smoothened (Smo) receptor is the major transducer of the Hedgehog (Hh) signaling pathway. On the basis of the structure of the acylthiourea Smo antagonist (MRT-10), a number of different series of analogous compounds were prepared by ligand-based structural optimization. The acylthioureas, originally identified as actives, were converted into the corresponding acylureas or acylguanidines. In each series, similar structural trends delivered potent compounds with IC50 values in the nanomolar range with respect to the inhibition of the Hh signaling pathway in various cell-based assays and of BODIPY-cyclopamine binding to human Smo. The similarity of their biological activities, in spite of discrete structural differences, may reveal the existence of hydrogen-bonding interactions between the ligands and the receptor pocket. Biological potency of compounds 61, 72, and 86 (MRT-83) were comparable to those of the clinical candidate GDC-0449. These findings suggest that these original molecules will help delineate Smo and Hh functions and can be developed as potential anticancer agents.
-
AMIDE DERIVATIVES FOR THE TREATMENT OF DISEASES MEDIATED BY CYTOKINES申请人:AstraZeneca AB公开号:EP1017378B1公开(公告)日:2002-12-11
-
BENZAMIDE DERIVATIVES AND THER USE AS CYTOKINE INHIBITORS申请人:AstraZeneca AB公开号:EP1115707B1公开(公告)日:2003-11-12
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫