2,6-二甲基哌啶 | 504-03-0
中文名称
2,6-二甲基哌啶
中文别名
二甲哌啶
英文名称
2.6-dimethylpiperidine
英文别名
2,6-Dimethylpiperidin;2,6-Dimethylpiperidine
CAS
504-03-0
化学式
C7H15N
mdl
MFCD00005986
分子量
113.203
InChiKey
SDGKUVSVPIIUCF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:127-128 °C768 mm Hg(lit.)
-
密度:0.84 g/mL at 25 °C(lit.)
-
闪点:53 °F
-
溶解度:溶于二甲基亚砜
-
保留指数:834;873;833
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,并应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:F,Xi,C
-
安全说明:S16,S26,S36/37/39
-
危险类别码:R34,R36/37/38,R11
-
WGK Germany:3
-
海关编码:2933399090
-
危险品运输编号:UN 1993 3/PG 2
-
RTECS号:OK5775000
-
包装等级:II
-
危险类别:3
-
危险性防范说明:P242,P264,P271,P280,P301+P330+P331,P304+P340,P305+P351+P338,P310,P363,P370+P378,P403+P233,P501
-
危险性描述:H225,H314
-
储存条件:密封保存,并置于通风、干燥的环境中。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2,6-Dimethylpiperidine
Synonyms: 2,6-Lupetidine
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,6-Dimethylpiperidine
CAS number: 504-03-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H15N
Molecular weight: 113.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2,6-Dimethylpiperidine
Synonyms: 2,6-Lupetidine
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,6-Dimethylpiperidine
CAS number: 504-03-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H15N
Molecular weight: 113.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-氯-2,6-二甲基哌啶 N-chloro-2,6-dimethylpiperidine 6830-30-4 C7H14ClN 147.648 1-氨基-2,6-二甲基哌啶 1-amino-2,6-dimethylpiperidine 39135-39-2 C7H16N2 128.217 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2,6-trimethyl-piperidine 669-81-8 C8H17N 127.23 1-氯-2,6-二甲基哌啶 N-chloro-2,6-dimethylpiperidine 6830-30-4 C7H14ClN 147.648 —— 1-fluoro-2,6-dimethyl-piperidine 24151-83-5 C7H14FN 131.193 1-氨基-2,6-二甲基哌啶 1-amino-2,6-dimethylpiperidine 39135-39-2 C7H16N2 128.217 —— 4-(2,6-dimethyl-1-piperidyl)butanol —— C11H23NO 185.31 —— 4-(2,6-dimethylpiperidino)-butyronitrile —— C11H20N2 180.293 —— 3-(2,6-dimethyl-piperidin-1-yl)-propan-1-ol 79156-90-4 C10H21NO 171.283 —— 1-(3-chloro-propyl)-2,6-cis-dimethyl-piperidine 79156-91-5 C10H20ClN 189.728 —— cis-2-(2,6-dimethyl-1-piperidinyl)ethylamine 769-24-4 C9H20N2 156.271 —— 2-(2,6-dimethylpiperidinyl)ethanol 23502-32-1 C9H19NO 157.256
反应信息
-
作为反应物:参考文献:名称:Metal-Free Hydrogenation of N-Based Heterocycles摘要:Metal-free hydrogenation of substituted pyridines, quinolines, and several other N-heterocycles is achieved upon treatment of the nitrogen-based Lewis base with an equivalent of the Lewis acid B(C6F5)(3) and H-2 (4 atm) at 115 degrees C, to afford the ammonium-[HB(C6F5)(3)] salts of the reduced N-heterocycles.DOI:10.1021/om4000727
-
作为产物:描述:1-甲基己-5-烯基胺 在 (CH2)2[N(2,6-diethylphenyl)]2 yttrium(III)N(trimethylsilyl)2 作用下, 生成 2,6-二甲基哌啶参考文献:名称:在钇和钕酰胺基络合物催化的分子内烯烃加氢胺化反应中,通过螯合二酰胺配位,可异常提高速率并改善非对映选择性。摘要:DOI:10.1002/1521-3773(20021004)41:19<3645::aid-anie3645>3.0.co;2-f
-
作为试剂:描述:参考文献:名称:Levy, Jeffrey N.; Mckenna, Charles E., Phosphorus, Sulfur and Silicon and the Related Elements, 1993, vol. 85, # 1-4, p. 1 - 8摘要:DOI:
文献信息
-
[EN] GALNAC PHOSPHORAMIDITES, NUCLEIC ACID CONJUGATES THEREOF AND THEIR USE<br/>[FR] PHOSPHORAMIDITES GALNAC, LEURS CONJUGUÉS D'ACIDES NUCLÉIQUES ET LEUR UTILISATION申请人:HOFFMANN LA ROCHE公开号:WO2016055601A1公开(公告)日:2016-04-14This invention generally relates to the field of phosphoramidite derivatives. In particular, the invention relates to N-Acetylgalactosamine phosphoramidite molecules and to conjugates of nucleic acid molecules with N-Acetylgalactosamine containing molecules. Also provided are methods for preparation of these molecules and possible uses thereof, in particular in medicine.
-
[EN] SULFONYLATED TETRAHYDROAZOLOPYRAZINES AND THEIR USE AS MEDICINAL PRODUCTS<br/>[FR] TÉTRAHYDROAZOLOPYRAZINES SULFONYLÉES ET LEUR UTILISATION EN TANT QUE PRODUITS MÉDICAMENTEUX申请人:GRUENENTHAL GMBH公开号:WO2010099938A1公开(公告)日:2010-09-10The present invention relates to sulfonylated tetrahydroazolopyrazines, methods for the preparation thereof, medicinal products containing these compounds and the use of substituted indole compounds for the preparation of medicinal products (Formula I).
-
[EN] UREA DERIVATIVES OF AMPHOTERICIN B DERIVED FROM SECONDARY AMINES<br/>[FR] DÉRIVÉS D'URÉE DE L'AMPHOTÉRICINE B DÉRIVÉE D'AMINES SECONDAIRES申请人:UNIV ILLINOIS公开号:WO2016112243A1公开(公告)日:2016-07-14Provided are certain urea derivatives of amphotericin B (AmB) having improved therapeutic index compared to AmB. The compounds of the invention are less toxic than AmB and are useful to treat fungal infections. In certain embodiments the urea derivative of AmB is a compound represented by formula (I) or a pharmaceutically acceptable salt thereof: wherein, independently for each occurrence: R represents methyl, ethyl, propyl, or isopropyl; R' represents methyl, ethyl, propyl, or isopropyl; or R and R', taken together with the nitrogen atom to which they are attached, represent a radical of a cyclic secondary amine. Also provided are methods for making the urea derivatives of AmB.
-
A Manganese Pre-Catalyst: Mild Reduction of Amides, Ketones, Aldehydes, and Esters作者:Colin M. Kelly、Robert McDonald、Orson L. Sydora、Mark Stradiotto、Laura TurculetDOI:10.1002/anie.201709441日期:2017.12.11Hey Man(ganese): A newly prepared (N-phosphinoamidinate)manganese pre-catalyst (see Scheme) has been shown to be effective for the hydrosilative reduction of a diverse scope of carbonyl compounds, and in most cases can be used at room temperature. The reaction proceeds under reaction conditions which are competitive with the most effective transition-metal catalysts known for such transformations,
-
[EN] INHIBITOR OF INDOLEAMINE-2,3-DIOXYGENASE (IDO)<br/>[FR] INHIBITEUR DE L'INDOLÉAMINE-2,3-DIOXYGÉNASE (IDO)申请人:INVENTISBIO INC公开号:WO2017139414A1公开(公告)日:2017-08-17The present disclosure provides compounds of Formula (I). The compounds described herein may be useful in treating a disease associated with IDO, for example, cancer or an infectious disease (e.g., viral or bacterial infectious diseases). Also, provided in the present disclosure are pharmaceutical compositions, kits, methods, and uses including or using a compound described herein.本公开提供了式(I)的化合物。本文描述的化合物可能在治疗与IDO相关的疾病方面有用,例如癌症或传染病(例如病毒或细菌感染性疾病)。本公开还提供了包括或使用本文描述的化合物的药物组合物、试剂盒、方法和用途。
表征谱图
-
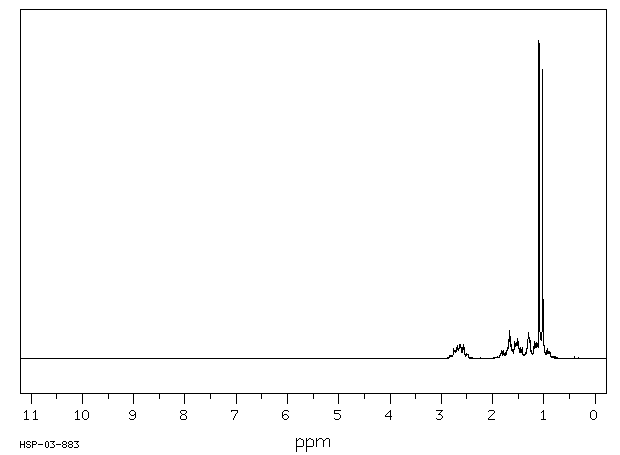
氢谱1HNMR
-
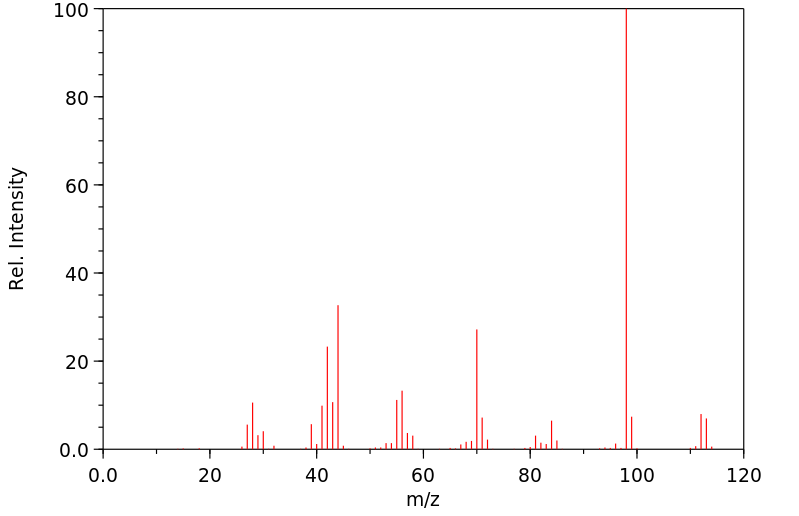
质谱MS
-
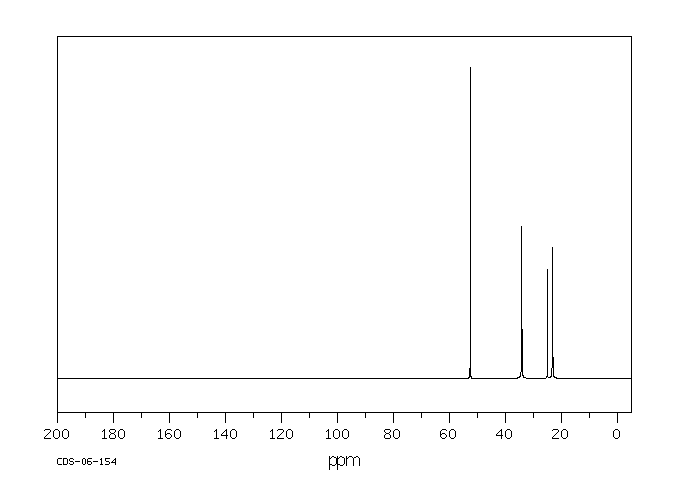
碳谱13CNMR
-
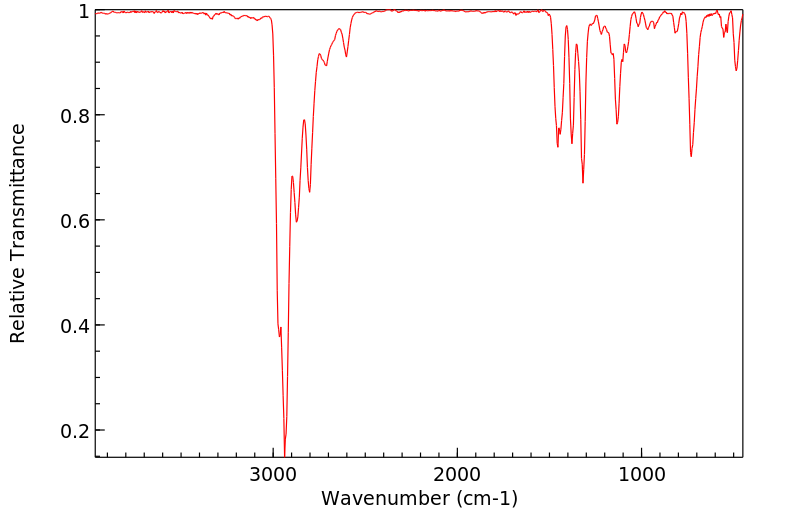
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺