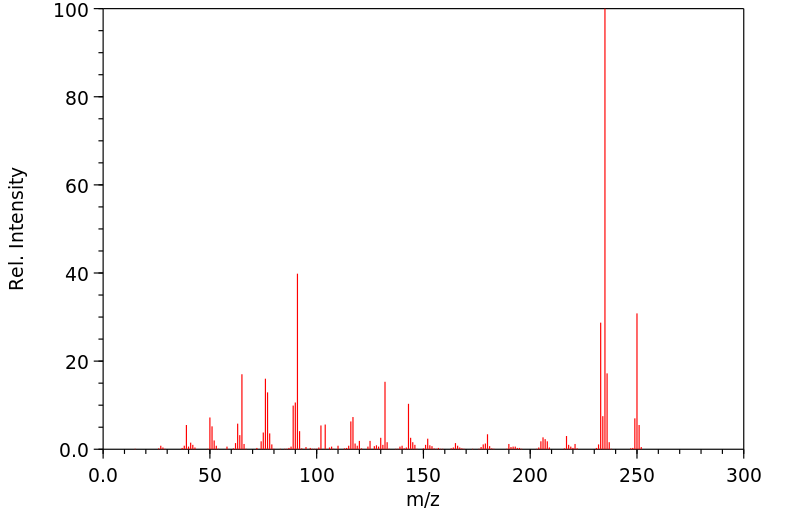
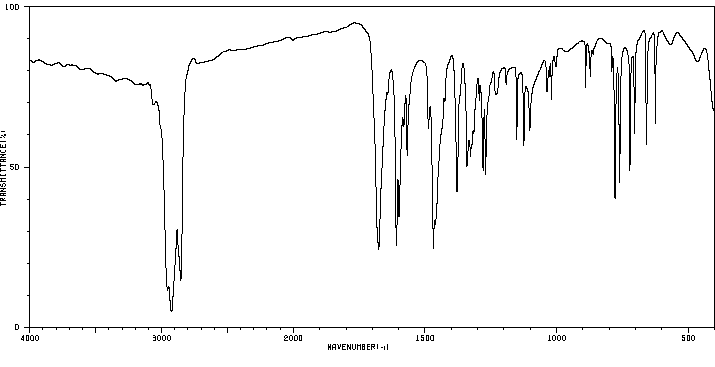
代谢
MORE THAN 99% OF THE DRUG IS METABOLIZED BY THE HEPATIC MICROSOMAL SYSTEM, AND 4-HYDROXYMETHAQUALONE AND THE N'-OXIDE ARE THE MAJOR PRIMARY METABOLITES. AT LEAST EIGHT OTHER HYDROXYL METABOLITES ARE FORMED.
来源:Hazardous Substances Data Bank (HSDB)