N-(p-methylbenzylidene)-p-nitroaniline | 20192-50-1
中文名称
——
中文别名
——
英文名称
N-(p-methylbenzylidene)-p-nitroaniline
英文别名
p-nitro p'-methyl benzilidine aniline;4-Nitro-4'-methylbenzylidene aniline;N-(4-methyl-benzylidene)-4-nitro-aniline;N-(4-Methyl-benzyliden)-4-nitro-anilin;N-(4-Methyl-benzal)-4-nitro-anilin;p-Toluylaldehyd-(4-nitro-anil);N-(4-methylbenzylidene)-4-nitroaniline;1-(4-methylphenyl)-N-(4-nitrophenyl)methanimine
CAS
20192-50-1
化学式
C14H12N2O2
mdl
——
分子量
240.261
InChiKey
PZHYCUFBBDDVKW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:2378
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:18
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:58.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2921420090
SDS
反应信息
-
作为反应物:描述:N-(p-methylbenzylidene)-p-nitroaniline 、 苯丙酮 在 三氟甲磺酸 、 silver trifluoromethanesulfonate 作用下, 以 甲苯 为溶剂, 反应 12.0h, 生成 2-p-tolyl-3-methyl-4-phenyl-6-nitroquinoline参考文献:名称:Silver-catalyzed one-step synthesis of multiply substituted quinolines摘要:A silver-catalyzed formation of C-C bond for the construction of a series of polysubstituted quinolines from arylamines, aldehydes, and ketones or arylamines and 1,3-diketones has been developed. The transformation is effective for a broad range of substrates, thus enabling the expansion of constituent architectures on the heterocyclic framework. This use of a single catalytic system to mediate chemical transformations in a synthetic operation is important for the development of new atom-economic strategies and this strategy is efficient in building complex structures from simple starting materials in an environmentally benign fashion. (C) 2015 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2015.12.028
-
作为产物:描述:(4-甲基苯基)甲醇 、 4-硝基苯胺 在 potassium hydroxide 作用下, 以 甲苯 为溶剂, 反应 30.0h, 以99%的产率得到N-(p-methylbenzylidene)-p-nitroaniline参考文献:名称:双异质催化剂Pd – [电子邮件保护](II)-MOF,用于从醇和胺中一步一步合成亚胺。摘要:一种新的Mn(II)金属-有机骨架(MOF)1是通过4,4,4-三氟-1-(4-(吡啶-4-基)苯基)丁烷-1,3-二酮(L)和Mn(OAc)2的溶液。图1的特征是包含蜂窝状通道的三重互穿NbO网,其中相对的Mn(II)···Mn(II)距离为23.5075(10)。此外,1可能是支撑Pd-Au双金属合金纳米颗粒以生成Pd- [受电子邮件保护](II)-MOF的复合催化体系的理想平台(2)。2个 可以是一种高活性的双功能多相催化剂,用于从苄醇和苯胺以及苄醇和苄胺一键串联合成亚胺。DOI:10.1021/acs.inorgchem.6b02592
文献信息
-
A facile and effective synthesis of 2-azetidinones via phosphonitrilic chloride作者:Maaroof ZareiDOI:10.1016/j.tet.2013.05.121日期:2013.8The Staudinger reaction of imines to β-lactams was successfully achieved with substituted acetic acid and phosphonitrilic chloride in one-pot under mild conditions. Several types of β-lactams, especially 3-electron-withdrawing group β-lactams, can be synthesized by this versatile and efficient method in good to excellent yields. This method is simple, clean, and the by-products were removed by simple
-
Hydrogen-Bonding Asymmetric Metal Catalysis with α-Amino Acids: A Simple and Tunable Approach to High Enantioinduction作者:Yingdong Lu、Tim C. Johnstone、Bruce A. ArndtsenDOI:10.1021/ja904185b日期:2009.8.19While asymmetric transition-metal catalysis has become a powerful method for constructing chiral products, a challenge in this field is the identification of the correct ligand for high selectivity. We report here a simple approach to chiral catalyst formation: coupling of an available pool of Brønsted acids, namely, amino acid derivatives, with tunable ligands on copper catalysts. This system can
-
Substituent Cross-Interaction Effects on the Electronic Character of the CN Bridging Group in Substituted Benzylidene Anilines − Models for Molecular Cores of Mesogenic Compounds. A <sup>13</sup>C NMR Study and Comparison with Theoretical Results作者:Helmi Neuvonen、Kari Neuvonen、Ferenc FülöpDOI:10.1021/jo0600508日期:2006.4.1could be verified. The electronic effects of the neighboring aromatic ring substituents systematically modify the sensitivity of the CN group to the electronic effects of the benzylidene or aniline ring substituents. Electron-withdrawing substituents on the aniline ring decrease the sensitivity of δC(CN) to the substitution on the benzylidine ring, while electron-donating substituents have the opposite在CDCl 3中,对一系列介晶分子模型化合物(即C 13)测量了13 C NMR化学位移δC (C N)。取代的亚苄基苯胺p -X C 6 H 4 CH NC 6 H 4 p -Y(X = NO 2,CN,CF 3,F,Cl,H,Me,MeO或NMe 2; Y = NO 2,CN ,F,Cl,H,Me,MeO或NMe 2)。δ的取代基依赖性Ç(CN)被用作研究电子取代基对偶氮甲碱单元的作用的工具。亚苄基取代基X有δ的反向效应Ç(C N):吸电子原因屏蔽的取代基,而给电子性的人的行为相反,感应效果清楚地在共振效应为主。相反,苯胺取代基Y发挥正常作用:吸电子取代基引起屏蔽,而供电子取代基引起C N碳屏蔽,感应效应和共振效应的强度非常相似。此外,可以验证X和Y之间是否存在特定的交叉相互作用。相邻芳环取代基的电子效应可系统地改变C的灵敏度N基团对亚苄基或苯胺环取代基的电子作用。吸电子苯胺环上的取代基降低δ的灵敏度Ç(C
-
Grinding Synthesis of Schiff Bases Combined with Infrared Irradiation作者:Jian-Ying Tong、Na-Bo Sun、Hong-Ke WuDOI:10.14233/ajchem.2013.14382日期:——Solid-phase synthesis combined with infrared irradiation promoted the formation of a series of Schiff bases in the condensation reaction between substituted benzaldehydes and anilines, in the solvent free. Benzaldehydes and anilines, containing either electron withdrawing or electron-releasing groups, were evaluated their substituent effect on the formation of the Schiff bases. Moreover, this new procedure is environmentally benign because no solvent was employed in the transformations.
-
Triarylmethyl Cation‐Catalyzed Three‐Component Coupling for the Synthesis of Unsymmetrical Bisindolylmethanes作者:William J. Patterson、Kelly Lucas、Vanessa A. Jones、Zhenghua Chen、Kevin Bardelski、Melissa Guarino‐Hotz、Cheyenne S. BrindleDOI:10.1002/ejoc.202100946日期:2021.12.28cations catalyze the synthesis of unsymmetrical bisindolylmethanes from imines and two different indoles. Optimization of the catalyst by tuning cation stability allows for excellent single addition selectivity. The single addition intermediates can be isolated or used in situ in a high-yielding one-pot two-step reaction to generate unsymmetrical bisindolylmethanes.
表征谱图
-
氢谱1HNMR
-
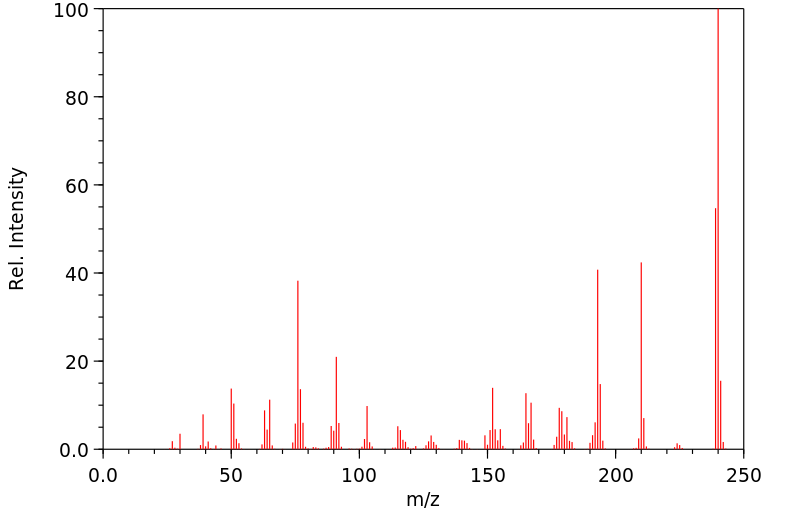
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫