HEPA | 52839-87-9
中文名称
——
中文别名
——
英文名称
HEPA
英文别名
(+/-)-2-hydroxy-2-phenyl-butyric acid amide;2-Hydroxy-2-phenyl-buttersaeure-amid;DL-2-hydroxy-2-phenyl butyramide;(+/-)-2-hydroxy-2-phenylbutyramide;2-Hydroxy-2-phenylbutyramide;2-hydroxy-2-phenylbutanamide
CAS
52839-87-9
化学式
C10H13NO2
mdl
——
分子量
179.219
InChiKey
KHJLUGZFVDFOKZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:93-95 °C
-
沸点:358.5±35.0 °C(Predicted)
-
密度:1.160±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:63.3
-
氢给体数:2
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis and Structure−Activity Relationship Studies for Hydantoins and Analogues as Voltage-Gated Sodium Channel Ligands摘要:We previously developed a preliminary 3-D QSAR model for the binding of 14 hydantoins to the neuronal voltage-gated sodium channel; this model was successful in designing,an effective non-hydantoin ligand. To further understand structural features that result in optimum binding. here we synthesized a variety of compound classes and evaluated their binding affinities to the neuronal voltage-gated sodium channel using the [H-3]-batrachotoxinin A 20-a-benzoate ([H-3]BTX-B) binding assay. In order to understand the importance of the hydantoin ring for good sodium channel binding, related non-hydantoins such as hydroxy amides, oxazolidinediones, hydroxy acids, and amino acids were included. Two major conclusions were drawn: (1) The hydantoin ring is not critical for compounds with long alkyl side chains, but it is important for compounds with shorter side chains. (2) Relative to Khodorov's pharmacophore. which contains two hydrophobic regions, a third hydrophobic region may enhance binding to provide nanomolar inhibitors.DOI:10.1021/jm040077o
-
作为产物:描述:2-羟基-2-乙基-2-苯基乙腈 在 盐酸 作用下, 以2.03 g的产率得到HEPA参考文献:名称:Synthesis and Structure−Activity Relationship Studies for Hydantoins and Analogues as Voltage-Gated Sodium Channel Ligands摘要:We previously developed a preliminary 3-D QSAR model for the binding of 14 hydantoins to the neuronal voltage-gated sodium channel; this model was successful in designing,an effective non-hydantoin ligand. To further understand structural features that result in optimum binding. here we synthesized a variety of compound classes and evaluated their binding affinities to the neuronal voltage-gated sodium channel using the [H-3]-batrachotoxinin A 20-a-benzoate ([H-3]BTX-B) binding assay. In order to understand the importance of the hydantoin ring for good sodium channel binding, related non-hydantoins such as hydroxy amides, oxazolidinediones, hydroxy acids, and amino acids were included. Two major conclusions were drawn: (1) The hydantoin ring is not critical for compounds with long alkyl side chains, but it is important for compounds with shorter side chains. (2) Relative to Khodorov's pharmacophore. which contains two hydrophobic regions, a third hydrophobic region may enhance binding to provide nanomolar inhibitors.DOI:10.1021/jm040077o
文献信息
-
Dl-hydroxy-alkyl-phenylamides having anticonvulsive activity申请人:Meza Toledo Enrique Sergio公开号:US20060287397A1公开(公告)日:2006-12-21New anticonvulsant compounds were synthesized and they include the compounds: DL-2-hydroxy-2-(3′,5′-bistrifluoromethylphenyl)butyramide, DL-2-hydroxy-2-(4′-trifluoromethylphenyl)butyramide, DL-2-hydroxy-2-(3′,4′-dichlorophenyl)butyramide, DL-2-hydroxy-2-(3′-bromophenyl)butyramide, DL-2-hydroxy-2-(4′-bromophenyl)butyramide, DL-2-hydroxy-2-(3′-nitrophenyl)butyramide, DL-3-hydroxy-3-(3′,4′-dichlorophenyl)pentanamide, DL-3-hydroxy-3-(4′-bromophenyl)pentanamide, DL-4-hydroxy-4-(3′,4′-dichlorophenyl) hexanamide and DL-4-hydroxy-4-(4′-bromophenyl)hexanamide. They have a significant anticonvulsant activity against pentylenetetrazol-induced seizures as well as unexpected differences in anticonvulsant activity respect those of the non-halogenated compounds. The invention further provides methods for the synthesis of the DL-hydroxy-alkyl-phenyl amides as exemplified in the examples.新的抗惊厥化合物已经合成,它们包括以下化合物:DL-2-羟基-2-(3′,5′-双三氟甲基苯基)丁酰胺,DL-2-羟基-2-(4′-三氟甲基苯基)丁酰胺,DL-2-羟基-2-(3′,4′-二氯苯基)丁酰胺,DL-2-羟基-2-(3′-溴苯基)丁酰胺,DL-2-羟基-2-(4′-溴苯基)丁酰胺,DL-2-羟基-2-(3′-硝基苯基)丁酰胺,DL-3-羟基-3-(3′,4′-二氯苯基)戊酰胺,DL-3-羟基-3-(4′-溴苯基)戊酰胺,DL-4-羟基-4-(3′,4′-二氯苯基)己酰胺和DL-4-羟基-4-(4′-溴苯基)己酰胺。它们对戊巴比妥诱导的癫痫发作具有显著的抗惊厥活性,同时与非卤代化合物相比具有意想不到的抗惊厥活性差异。该发明还提供了合成DL-羟基-烷基-苯胺的方法,如示例中所示。
-
Phenyl alcohol amides having anticonvulsant activity申请人:——公开号:US05463125A1公开(公告)日:1995-10-31New anticonvulsant compounds include (.+-.)-2-hydroxy-2-phenylbutyramide and (.+-.)-3-hydroxy-3-phenylpentamide. These homologues of (.+-.)-4-hydroxy-4-phenylhexanamide have anticonvulsant activity as well as unexpected properties, particularly low neurotoxicity and pharmacological differences. The invention further provides methods for the synthesis of (.+-.)-2-hydroxy-2-phenylbutyramide and (.+-.)-3-hydroxy-3-phenylpentamide as exemplified in the examples.新的抗惊厥化合物包括(.+-.)-2-羟基-2-苯基丁酰胺和(.+-.)-3-羟基-3-苯基戊酰胺。这些与(.+-.)-4-羟基-4-苯基己酰胺同系物具有抗惊厥活性,以及意外的特性,特别是低神经毒性和药理学差异。该发明还提供了合成(.+-.)-2-羟基-2-苯基丁酰胺和(.+-.)-3-羟基-3-苯基戊酰胺的方法,如示例所示。
-
[EN] SUBTYPE-SELECTIVE NMDA RECEPTOR LIGANDS AND THE USE THEREOF<br/>[FR] LIGANDS DU RECEPTEUR N-METHYL-D-ASPARTATE SELECTIFS DE SOUS-TYPE申请人:STATE OF OREGON, acting by and through THE OREGON STATE BOARD OF HIGHER EDUCATION, acting for and on behalf of THE OREGON HEALTH SCIENCES UNIVERSITYA ND THE UNIVERSITY OF OREGON, EUGENE OREGON公开号:WO1997023202A1公开(公告)日:1997-07-03(EN) The invention relates to subtype-selective NMDA receptor ligands and the use thereof for treating or preventing neuronal loss associated with stroke, ischemia, CNS trauma, hypoglycemia and surgery, as well as treating neurodegenerative diseases including Alzheimer's disease, amyotrophic lateral sclerosis, Huntington's diseae, Parkinson's disease and Down's syndrome, treating or preventing the adverse consequences of the overstimulation of the excitatory amino acids, treating anxiety, psychosis, convulsions, chronic pain, glaucoma, CMV retinitis, urinary incontinence and inducing anesthesia, as well as for enhancing cognition, treating or preventing opiate tolerance, treating or preventing aminoglycoside antibiotic induced hearing loss, and treating opiate withdrawal.(FR) Cette invention a trait à des ligands du récepteur N-méthyl-D-aspartate (NMDA) sélectifs de sous-type et à leur utilisation dans le traitement ou la prévention de la dépopulation neuronale associée à un ictus, une ischémie, une lésion du système nerveux central, une hypoglycémie et à un acte chirurgical, ainsi que dans le traitement de maladies neurodégénérescentes, au nombre desquelles la maladie d'Alzheimer, la sclérose latérale amyotrophique, les maladies d'Huntington et de Parkinson ainsi que le syndrome de Down. Ces ligands sont également utilisés dans le traitement et la prévention des conséquences préjudiciables d'une stimulation excessive d'acides aminés excitateurs, le traitement de l'anxiété, de psychoses, de convulsions, de douleurs chroniques, du glaucome, de la rétinite à cytomégalovirus, de l'incontinence urinaire et pour le déclenchement de l'anesthésie ainsi que comme tonique cérébro-actif. Ces ligands sont, en outre, utilisés dans le traitement et la prévention de l'accoutumance opiacée et le traitement du sevrage des dépendances aux opiacés ainsi que pour le traitement et la prévention du déficit auditif provoqué par des aminoglycosides.
-
Hydroquinonylphenyl butyric acid amide derivative申请人:SUNTORY LIMITED公开号:EP0298758A2公开(公告)日:1989-01-11A hydroquinonylphenyl butyric acid amide derivative having the formula (I): wherein R¹ represents an aromatic or heterocyclic group which may be substituted, R² represents a hydrogen atom, a lower alkylcarbonyl group, an aromatic carbonyl or heterocyclic carbonyl group which may be substituted, and X represents an oxygen atom or sulfur atom or a pharmaceutically acceptable salt thereof, which has a cerebral insufficiency improving activity.
-
Pyridylethylated Oxazolidinediones. II<sup>1</sup>作者:Seymour L. Shapiro、Ira M. Rose、Eric Roskin、Louis FreedmanDOI:10.1021/ja01511a028日期:1959.1
表征谱图
-
氢谱1HNMR
-
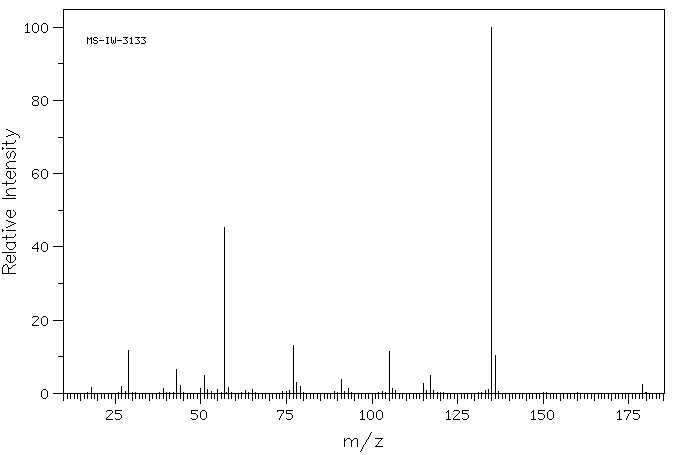
质谱MS
-
碳谱13CNMR
-
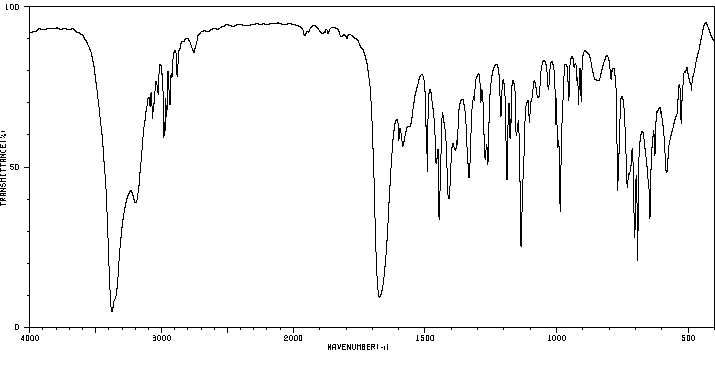
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫