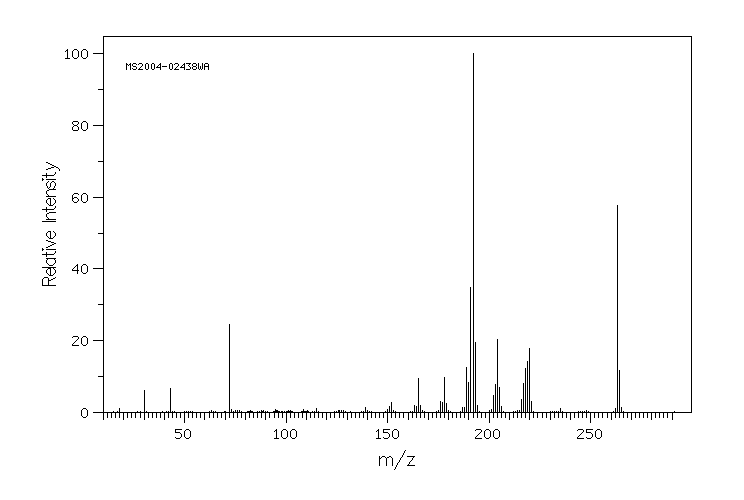
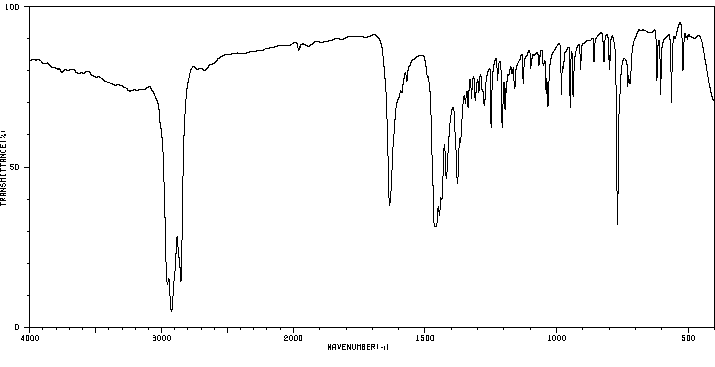
6-acetyl-5,6,6a,7-tetrahydro-4H-dibenzo(de,g)quinoline | 14528-29-1
中文名称
——
中文别名
——
英文名称
6-acetyl-5,6,6a,7-tetrahydro-4H-dibenzo(de,g)quinoline
英文别名
6-acetyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline;1-(5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-6-yl)ethanone
CAS
14528-29-1
化学式
C18H17NO
mdl
——
分子量
263.339
InChiKey
AZKNTZGHGOBKGF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:20
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.28
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:1
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 阿朴啡 (+/-)-Aporphine 478-57-9 C17H17N 235.329 —— (-)-Aporphine —— C17H17N 235.329 5,6,6A,7-四氢-4H-二苯并[去,G]喹啉 aporphine 519-01-7 C16H15N 221.302
反应信息
-
作为反应物:参考文献:名称:A convenient formation of aporphine core via benzyne chemistry: conformational analysis and synthesis of (R)-aporphine摘要:Total synthesis of (R)-aporphine has been accomplished by an approach that employs in the key step a sequence of transformations involving a [4+2] cycloaddition reaction followed by a hydrogen migration, leading to aporphine core in good yield, which was subjected to a 1D gradient NOE experiment, conformational analysis, and simple transformations, including a small scale resolution process, to afford enantiomericallyenriched aporphine alkaloid. (C) 2015 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2015.10.083
-
作为产物:描述:N-(2-苯乙基)乙酰胺 在 吡啶 、 cesium fluoride 作用下, 以 乙腈 为溶剂, 反应 30.5h, 生成 6-acetyl-5,6,6a,7-tetrahydro-4H-dibenzo(de,g)quinoline参考文献:名称:A convenient formation of aporphine core via benzyne chemistry: conformational analysis and synthesis of (R)-aporphine摘要:Total synthesis of (R)-aporphine has been accomplished by an approach that employs in the key step a sequence of transformations involving a [4+2] cycloaddition reaction followed by a hydrogen migration, leading to aporphine core in good yield, which was subjected to a 1D gradient NOE experiment, conformational analysis, and simple transformations, including a small scale resolution process, to afford enantiomericallyenriched aporphine alkaloid. (C) 2015 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2015.10.083
文献信息
-
Aporphine Alkaloid Synthesis and Diversification via Direct Arylation作者:Marc Lafrance、Nicole Blaquiere、Keith FagnouDOI:10.1002/ejoc.200600674日期:2007.2aporphines by reaction with benzodioxole, pyridine N-oxide and pyrazine N-oxide. Successful application of direct arylation in these diversification reactions highlights its utility not only in convergent, but also in divergent synthesis. We also describe enantioselective syntheses of (R)-nornuciferine and (R)-nuciferine employing a catalytic asymmetric transfer hydrogenation in high yield and excellent enantiomeric
-
[EN] NITRIC OXIDE AND ITS BIOMEDICAL SIGNIFICANCE<br/>[FR] OXYDE NITRIQUE ET SA VALEUR BIOMÉDICALE申请人:UNIV NEW YORK STATE RES FOUND公开号:WO2012078155A1公开(公告)日:2012-06-14A pharmaceutical composition for stimulating nitric oxide production in mammalian cells, the pharmaceutical composition including at least one compound selected from a group consisting of: 2,3-dihydroxypropyl oleate; bis(m-phenoxyphenyl) ether; 6-acetyl-5,6,6a,7-tetrahydro-4H-dibezo(de,g)quinoline; and (+)-N-(p-(2-methylbutoxy)benzylidene)-4-(2-methylbutyl)aniline.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
鹅掌楸碱
靛红
阿朴啡
阿朴啡
阿朴吗啡
阿扑吗啡
铁线蕨叶碱
醛基去甲海罂粟碱
载堇碱盐酸盐
诏松草卟吩
观音莲明
蝙蝠葛任碱
莲碱
荷叶碱
荷包牡丹酮
荷包牡丹碱
荷包牡丹碱
罂粟碱氢溴酸盐
绕袂碱; 莲碱; 罗默碱; 阿朴雷因
紫堇块茎碱
米什莱恩A
竹叶椒碱
空褐麟碱
盐酸波尔定碱
盐酸去水吗啡
白蓬草米宁
異蒂巴因
番荔枝碱
甲氧基铁线蕨叶碱
瓜馥木碱甲
海罂粟碱溴化氢
海罂粟碱
海罂粟碱
洋玉兰碱
波尔定碱 2-甲醚
波尔定碱
氧海罂粟碱甲碘化物
氧海罂粟碱
氧化紫番荔枝碱
毛叶含笑碱
木番荔枝碱盐酸盐
木番荔枝碱
木兰花碱(氯化物)
木兰花碱
暂无
无根藤辛
无根藤次碱
新木姜子素
放线瑞香宁
异阿扑吗啡