2-乙基己酸酐 | 36765-89-6
中文名称
2-乙基己酸酐
中文别名
——
英文名称
2-ethylhexanoic anhydride
英文别名
2-ethyl 1-hexanoic anhydride;2-ethylhexanoyl 2-ethylhexanoate
CAS
36765-89-6
化学式
C16H30O3
mdl
MFCD00043963
分子量
270.412
InChiKey
TVPCUVQDVRZTAL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:163 °C (16 mmHg)
-
密度:0.9909
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):5.6
-
重原子数:19
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2915900090
SDS
| Name: | 2-Ethylhexanoic anhydride, 95+% Material Safety Data Sheet |
| Synonym: | None. |
| CAS: | 36765-89-6 |
Synonym: None.
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 36765-89-6 | 2-ethylhexanoic anhydride | 95+ | 253-195-1 |
Risk Phrases: 36/37/38
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Irritating to eyes, respiratory system and skin.Moisture sensitive. Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
SECTION 4 - FIRST AID MEASURES
Eyes:
Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Do NOT get water inside containers. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Provide ventilation. Do not get water inside containers.
SECTION 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Use with adequate ventilation. Do not allow contact with water. Keep from contact with moist air and steam.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from moisture.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 36765-89-6: Personal Protective Equipment
Eyes:
Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: yellow orange
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 163 deg C @ 16.00 mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: > 212 deg C (> 413.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: negligible
Specific Gravity/Density: Not available.
Molecular Formula: C16H30O3
Molecular Weight: 270.40
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions. Moisture sensitive.
Conditions to Avoid:
Incompatible materials, moisture, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Moisture, strong oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 36765-89-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-ethylhexanoic anhydride - Not listed by ACGIH, IARC, or NTP.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material.
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 36765-89-6: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 36765-89-6 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 36765-89-6 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 9/02/1997 Revision #5 Date: 3/18/2003 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙基-己酸稀土盐 2-Ethylhexanoic acid 149-57-5 C8H16O2 144.214 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-ethyl-hexanoic acid isopropenyl ester 25309-43-7 C11H20O2 184.279
反应信息
-
作为反应物:参考文献:名称:Auf das Zentralnervensystem wirkende Substanzen XXIX。Überneuartige schwefelhaltige杂环素:4,4-二烷基-1,2-硫氮杂环丁烷-3-on-1,1-二氧化物和2,4,4-三烷基-1,2-硫氮杂环丁烷-3-on-1,1-二氧化物摘要:Es wird der Aufbau von 4,4-Dialkyl-1,2-thioazetidin-3-on-1,1-dioxiden beschrieben,die Erivs bisher praktisch unbekannten viergliedrigen Heterocyclus mit zwei benachbarten Heteroatomen sind。Diese Substanzen werden aus denα,α-Dialkylsulfonylessigsäure-dinatriumsalzenüberdie entsprechendenSäuredichloridemit Ammoniak erhalten。Neben der Synthese wirdüberchemische und physikalische Eigenschaften dieser neuenDOI:10.1002/hlca.19620450238
-
作为产物:描述:参考文献:名称:Auf das Zentralnervensystem wirkende Substanzen XXIX。Überneuartige schwefelhaltige杂环素:4,4-二烷基-1,2-硫氮杂环丁烷-3-on-1,1-二氧化物和2,4,4-三烷基-1,2-硫氮杂环丁烷-3-on-1,1-二氧化物摘要:Es wird der Aufbau von 4,4-Dialkyl-1,2-thioazetidin-3-on-1,1-dioxiden beschrieben,die Erivs bisher praktisch unbekannten viergliedrigen Heterocyclus mit zwei benachbarten Heteroatomen sind。Diese Substanzen werden aus denα,α-Dialkylsulfonylessigsäure-dinatriumsalzenüberdie entsprechendenSäuredichloridemit Ammoniak erhalten。Neben der Synthese wirdüberchemische und physikalische Eigenschaften dieser neuenDOI:10.1002/hlca.19620450238
-
作为试剂:描述:参考文献:名称:USE OF ACID ANHYDRIDE ACCELERANTS FOR THE PRODUCTION OF HIGH PURITY POLYOL ESTERS摘要:一种生产高纯度聚醇酯的方法包括将聚醇与线性或支链脂肪族单羧基C3-20酸的过量反应,酯化聚醇的总量不超过聚醇总量,形成具有羟值在7至约50毫克KOH/g之间的中间反应组成物。然后向中间反应组成物中添加相应的线性或支链脂肪族单羧基C3-20酸的酐,其量为中间组成物中可用OH的1至约2.5当量,形成反应混合物。将反应混合物加热5-30分钟,或直到所有相应的酐反应形成反应产物。然后对反应产物去酸。在上述任何反应步骤中,不存在任何浓度高于约15ppm的金属或酸催化剂或漂白剂。公开号:US20180194712A1
文献信息
-
GLYCERYL ASCORBIC ACID ACYLATED DERIVATIVE OR ITS SALT, PRODUCTION METHOD THEREOF, AND COSMETICS申请人:YOSHIOKA Masato公开号:US20130204017A1公开(公告)日:2013-08-08A glyceryl ascorbic acid acylated derivative or its salt, which has an ascorbic acid structure where 2- and/or 3-positions of the structure are substituted with glyceryl groups and some of the hydroxyl groups in the structure and/or in the glyceryl group are acylated, a production method of the glyceryl ascorbic acid acylated derivative and a cosmetic containing the glyceryl ascorbic acid acylated derivative or its salt are provided.
-
Ti-<i>Crossed</i>-Claisen Condensation between Carboxylic Esters and Acid Chlorides or Acids: A Highly Selective and General Method for the Preparation of Various β-Keto Esters作者:Tomonori Misaki、Ryohei Nagase、Kunshi Matsumoto、Yoo TanabeDOI:10.1021/ja043833o日期:2005.3.9condensation between a 1:1 mixture of carboxylic esters and acid chlorides promoted by TiCl4-Bu3N-N-methylimidazole proceeded successfully to give various beta-keto esters in good yields with excellent selectivities (19 examples, approximately 48-95% yield; cross/self-selectivity = approximately 96/4-99/1). The present method was extended to the condensation between a 1:1 mixture of carboxylic acids and carboxylic
-
Oligonucleotide with protected base申请人:Ajinomoto Co., Inc.公开号:US09371353B2公开(公告)日:2016-06-21The present invention provides a protected nucleotide for elongation, which can be purified efficiently and in a high yield by a liquid-liquid extraction operation, and can achieve an oligonucleotide production method by a phosphoramidite method. It has been found that the above-mentioned problem can be solved by a particular oligonucleotide comprising a protected base and/or particular oligonucleotide protected by a branched chain-containing aromatic group at 3′-position.本发明提供了一种用于延伸的受保护核苷酸,可以通过液液萃取操作高效地和高产率地纯化,并且可以通过磷酰胺酯法实现寡核苷酸的生产方法。已经发现,上述问题可以通过包含受保护碱基和/或在3'-位置含有支链含芳基团的特定寡核苷酸来解决。
-
Nickel-Catalyzed Decarboxylative Alkylation of Aryl Iodides with Anhydrides作者:Hui Chen、Lu Hu、Wenzhi Ji、Licheng Yao、Xuebin LiaoDOI:10.1021/acscatal.8b03396日期:2018.11.2scope of aliphatic carboxylic anhydrides and tolerates synthetically useful aromatic substituents. Assisted by a redox system of pyridine N-oxide and zinc additives, the current reaction occurs under mild conditions and without the assistance of photocatalyst. Notably, this method features high chemoselectivity toward alkyl migration with mixed aliphatic/aromatic anhydrides. Thus, it provides a powerful
-
[EN] PROCESS FOR THE PRODUCTION OF DIACYL PEROXIDES<br/>[FR] PROCÉDÉ DE PRODUCTION DE PEROXYDES DE DIACYLE申请人:NOURYON CHEMICALS INT BV公开号:WO2020249688A1公开(公告)日:2020-12-17Process for the production of a diacyl peroxide involving the reaction of an anhydride with hydrogen peroxide, removal of the formed carboxylic acid, production of an anhydride from said carboxylic acid, and recycling of the anhydride within the process.
表征谱图
-
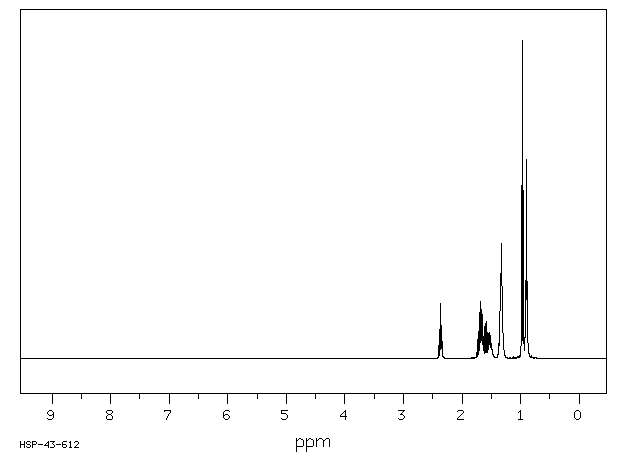
氢谱1HNMR
-
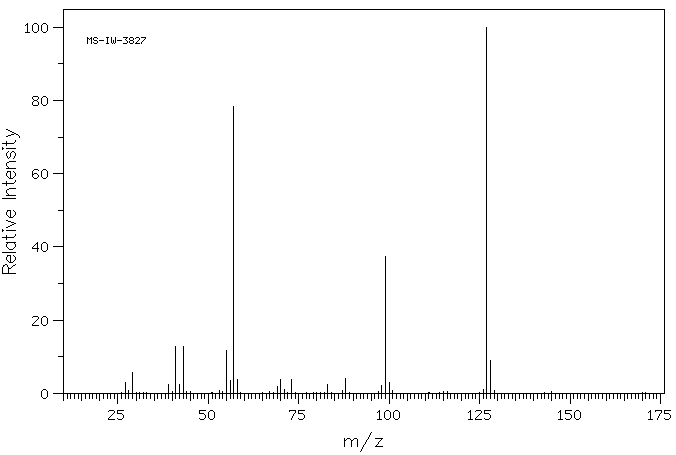
质谱MS
-
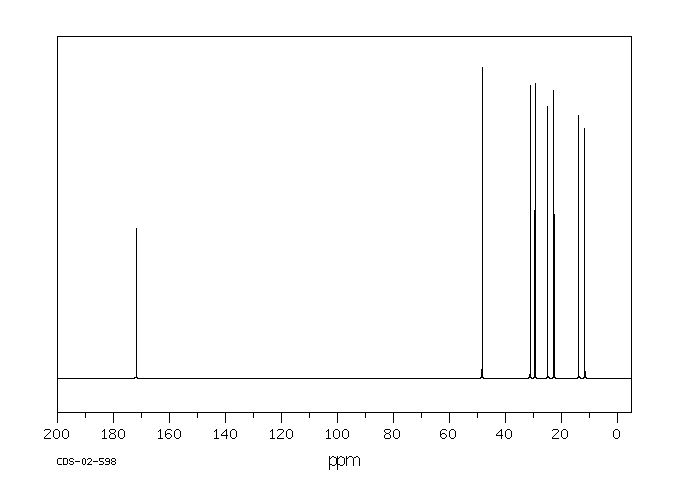
碳谱13CNMR
-
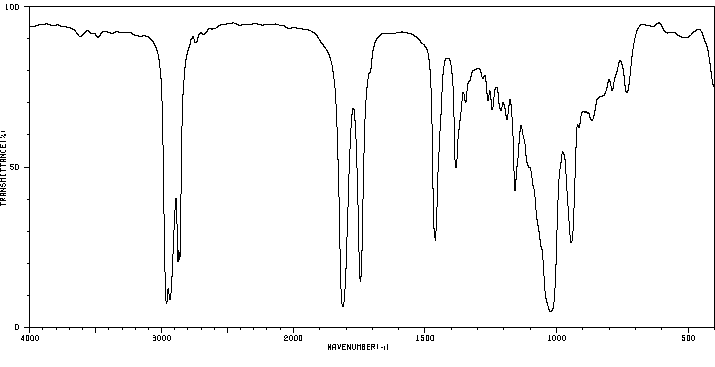
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸