2-十九烷醇 | 26533-36-8
中文名称
2-十九烷醇
中文别名
——
英文名称
2-nonadecanol
英文别名
nonadecan-2-ol;Methyl-heptadecyl-carbinol
CAS
26533-36-8
化学式
C19H40O
mdl
MFCD00046662
分子量
284.526
InChiKey
QXYWIOWTBOREMG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:52°C
-
沸点:357°C (estimate)
-
密度:0.8528 (estimate)
计算性质
-
辛醇/水分配系数(LogP):8.9
-
重原子数:20
-
可旋转键数:16
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905199090
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : Nonadecan-2-ol
CAS-No. : 26533-36-8
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008
Not a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C19H40O
Molecular Weight : 284,53 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing 49 - 51 °C
point
f) Initial boiling point and 166 °C at 1 hPa
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
反应信息
-
作为反应物:参考文献:名称:The Action of Sodium Cyanide on 1,3-Dibromo-1,3-dibenzoylpropane摘要:DOI:10.1021/ja01277a031
-
作为产物:描述:参考文献:名称:Dreger et al., Industrial and Engineering Chemistry, 1944, vol. 36, p. 611摘要:DOI:
文献信息
-
Design of Manganese Phenol Pi‐complexes as Shvo‐type Catalysts for Transfer Hydrogenation of Ketones作者:Nikita V. Shvydkiy、Oleksandr Vyhivskyi、Yulia V. Nelyubina、Dmitry S. PerekalinDOI:10.1002/cctc.201801797日期:2019.3.20Catalytic hydrogenation is one of the most important reactions both in academic research and industry. We explored ability of the manganese pi‐complexes to act as Shvo‐type catalysts for transfer hydrogenation of ketones. DFT calculations suggested that the transfer of hydrogen atoms from the hypothetical intermediate [(C6Me3H2OH)Mn(CO)2H] to acetone has low activation barrier of 10.9 kcal mol−1. Experimentally
-
NOVEL TETRACARBOXYLIC DIANHYDRIDE, POLYIMIDE RESIN AND METHOD FOR PRODUCING THE SAME, PHOTOSENSITIVE RESIN COMPOSITIONS, PATTERNING PROCESS, METHOD FOR FORMING CURED FILM, INTERLAYER INSULATING FILM, SURFACE PROTECTIVE FILM, AND ELECTRONIC PARTS申请人:SHIN-ETSU CHEMICAL CO., LTD.公开号:US20190169211A1公开(公告)日:2019-06-06The present invention has been made in view of the circumstances herein. An object of the present invention is to provide: a tetracarboxylic dianhydride which can lead to a polyimide usable as a base resin of a photosensitive resin composition capable of forming a fine pattern and obtaining high resolution without impairing excellent characteristics such as mechanical strength and adhesiveness; a polyimide resin obtained by using the tetracarboxylic dianhydride; and a method for producing the polyimide resin. The tetracarboxylic dianhydride is shown by the following general formula (1).
-
Sulfonated sugar compounds, pharmaceutical compositions which contain the same, and methods of treating tumors with the same申请人:Ohta Keisuke公开号:US20090209475A1公开(公告)日:2009-08-20Sulfoquinovosylacyl propanediol compounds represented by formula (I): wherein R 1 is an acyl residue of a fatty acid, Y is a number of 1, 2 or 3, and M represents a cation having a positive charge equal to Y and pharmaceutically acceptable salts thereof are effective for treating tumors.
-
NOVEL COMPOUND, POLYIMIDE RESIN AND METHOD OF PRODUCING THE SAME, PHOTOSENSITIVE RESIN COMPOSITION, PATTERNING METHOD AND METHOD OF FORMING CURED FILM, INTERLAYER INSULATING FILM, SURFACE PROTECTIVE FILM, AND ELECTRONIC COMPONENT申请人:International Business Machines Corporation公开号:US20210317270A1公开(公告)日:2021-10-14Provided is a compound that can be used as a base resin for a photosensitive resin composition. The photosensitive resin can form a fine pattern and can achieve high resolution without impairing mechanical strength and solubility. The compound is represented by the general formula (1): wherein Z represents a linear, branched or cyclic divalent hydrocarbon group having 2 to 30 carbon atoms; X 1 to X 3 represent any of —CO 2 —, —CONR X1 —, —O—, —NR X1 —, —S—, —SO 2 —, —SO 3 — and —SO 2 NR X1 — and may be the same as or different from each other, provided that R X1 is a hydrogen atom or a monovalent hydrocarbon group having 1 to 30 carbon atoms; Ar represents a divalent aromatic group having 2 to 30 carbon atoms; L 1 and L 2 independently represent a divalent hydrocarbon group having 1 to 30 carbon atoms; and x and y are each independently 0 or 1.
-
TETRACARBOXYLIC ACID DIESTER COMPOUND, POLYMER OF POLYIMIDE PRECURSOR AND METHOD FOR PRODUCING SAME, NEGATIVE PHOTOSENSITIVE RESIN COMPOSITION, PATTERNING PROCESS, AND METHOD FOR FORMING CURED FILM申请人:SHIN-ETSU CHEMICAL CO., LTD.公开号:US20180120702A1公开(公告)日:2018-05-03The present invention provides a tetracarboxylic acid diester compound represented by the following general formula (1), wherein, X 1 represents a tetravalent organic group, and R 1 represents a group represented by the following general formula (2), wherein, the dotted line represents a bonding, Y 1 represents an organic group with a valency of k+1, Rs represents a group containing at least one silicon atom, “k” represents 1, 2 or 3, and “n” represents 0 or 1. There can be provided a tetracarboxylic acid diester compound which can lead a polymer of a polyimide precursor capable of using a base resin of a negative photosensitive resin composition which is capable of forming a fine pattern and giving high resolution, a polymer of a polyimide precursor obtained by using the tetracarboxylic acid diester compound and a method for producing the same.
表征谱图
-
氢谱1HNMR
-
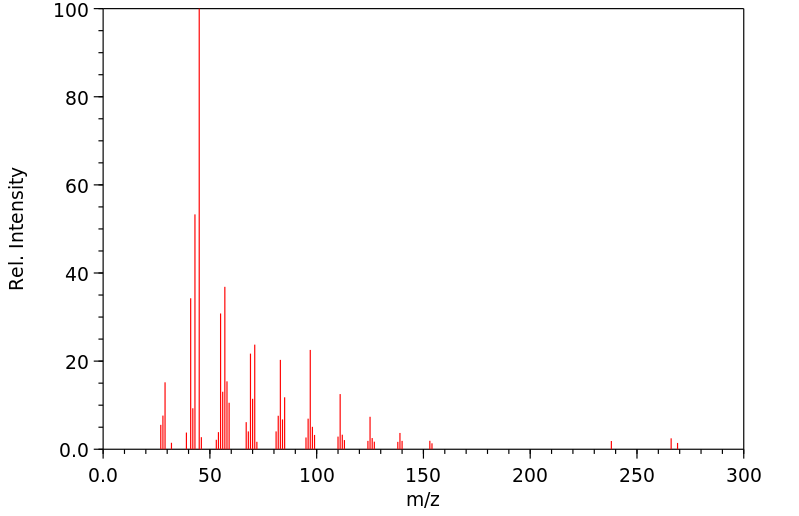
质谱MS
-
碳谱13CNMR
-
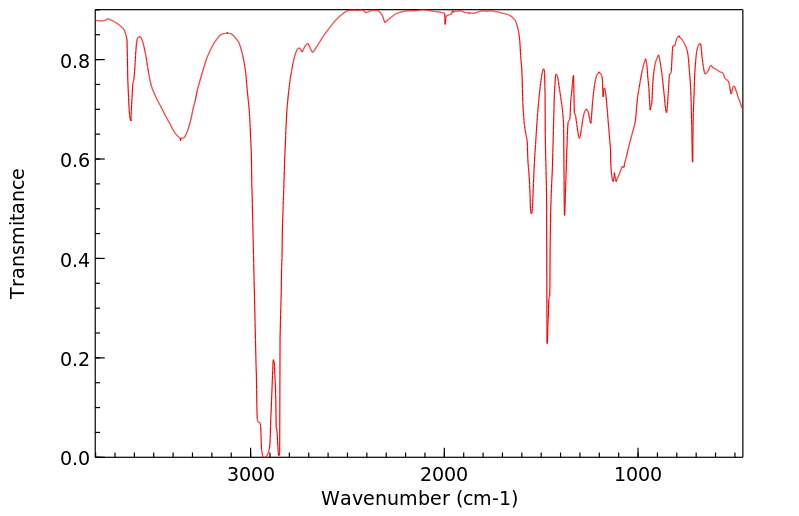
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯