2-氢过氧基丙烷 | 3031-75-2
中文名称
2-氢过氧基丙烷
中文别名
——
英文名称
2-hydroperoxypropane
英文别名
isopropyl hydroperoxide;Hydroperoxide, 1-methylethyl
CAS
3031-75-2
化学式
C3H8O2
mdl
——
分子量
76.0953
InChiKey
SGJUFIMCHSLMRJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:25°C
-
沸点:76.14°C (rough estimate)
-
密度:0.8923 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2909600000
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Inhibition of Warfarin Anticoagulation Associated with Chelation Therapy摘要:螯合疗法最初仅用于治疗重金属中毒的患者。现在一些医生正在将这种疗法用于多种病症,最常见的是冠状动脉心脏病。一名64岁的男性在接受螯合疗法后出现了华法林抗凝功能受损的情况。他的国际标准化比值(INR)从治疗前一天的2.6降至治疗后一天的1.6。螯合疗法是否降低华法林抗凝效果尚不确定。然而,由于这种潜在的相互作用,临床医生应考虑在接受螯合疗法的患者中增加INR监测。DOI:10.1592/phco.22.12.1067.33602
-
作为产物:描述:参考文献:名称:钴 (III)-烷基过氧配合物的合成、结构和反应性及其在碳氢化合物的化学计量和催化氧化中的作用摘要:尽管 Co(III)-烷基过氧物种经常被认为是用钴催化剂对烃类进行工业氧化的中间体,但离散 [LCoIII-OOR] 配合物的例子及其氧化能力的研究很少。在这项工作中,已经合成了 12 种具有两种不同配体 L 和各种一级、二级和三级 R 基团的此类配合物,其中 7 种已通过 X 射线晶体学表征。两个配体 N,N-bis[2-(2-pyridyl)ethyl]pyridine-2,6-dicarboxamide (Py3PH2, 1) 和 N,N-bis[2-(1-pyrazolyl) 的二价阴离子 (L2-) )乙基]吡啶-2,6-二甲酰胺 (PyPz2PH2, 2) 除了三个吡啶或一个吡啶和两个吡唑氮外,还以五齿方式与两个去质子化的羧酰胺氮结合 Co(III) 中心,以提供 [LCoIII(H2O)] 和 [LCoIII(OH)] 类型的复合物。[LCoIII(OH)] 复合物与 ROOHDOI:10.1021/ja9814873
-
作为试剂:参考文献:名称:纳米金催化烷基取代的苯和正构烷烃的选择性氧化摘要:我们报告了纳米多孔二氧化硅负载的金纳米粒子催化剂的合成及其对各种取代的烷基苯和线性烷烃的氧化反应的选择性和高效催化性能。通过使用负载的半缩醛基团作为还原剂,通过在半缩醛官能化的介孔二氧化硅的纳米孔内原位还原Au(III)离子来合成Au纳米颗粒。所得介孔二氧化硅负载的金纳米颗粒有效催化了不同烷基取代的苯和线性烷烃与t的氧化反应。丁基过氧化氢(TBHP)作为氧化剂。在某些情况下,催化反应的反应物转化率最高可达〜99%,对酮产物的选择性最高可达〜100%。催化剂对酮产物的高选择性是前所未有的,特别是考虑到在反应过程中仅使用温和的反应条件而没有使用添加剂的事实。DOI:10.1016/j.apcata.2012.05.029
文献信息
-
A new binuclear oxovanadium(v) complex as a catalyst in combination with pyrazinecarboxylic acid (PCA) for efficient alkane oxygenation by H2O2作者:Manas Sutradhar、Nikita V. Shvydkiy、M. Fátima C. Guedes da Silva、Marina V. Kirillova、Yuriy N. Kozlov、Armando J. L. Pombeiro、Georgiy B. Shul'pinDOI:10.1039/c3dt50584g日期:——[VO(OEt)(EtOH)}2L] (1) where H4L is bis(2-hydroxybenzylidene)terephthalohydrazide has been synthesized and fully characterized. The combination of 1 with pyrazine-2-carboxylic acid (PCA; a cocatalyst) affords a catalytic system for the efficient oxidation of saturated hydrocarbons, RH, with hydrogen peroxide and air in acetonitrile solution at 50 °C to produce alkyl hydroperoxides, ROOH, as the main一种新的双核氧钒(V)络合物[VO(OEt)(EtOH)} 2 L](1),其中H 4 L为双(2-羟基亚苄基)对苯二甲酰肼已经合成并充分表征。的组合1与吡嗪-2-羧酸 (PCA;助催化剂)提供了一种催化体系,可有效氧化饱和烃RH 过氧化氢并在50°C的乙腈溶液中通入空气,以产生烷基氢过氧化物ROOH,这是主要的主要产物。在该反应中已经获得了很高的周转率(TONs):例如,在2220分钟之后,TON = 44 000,初始TOF(周转频率)= 3300 h -1每个复合物1分子。在1 / PCA存在下,环己烷氧化的估计活化能为E a = 16±2 kcal mol -1。该值与由(n -Bu 4 N)[VO 3 ] / PCA组合催化的H 2 O 2环己烷氧化所得的值相同(17±2 kcal mol -1)。已经确定了初始氧化速率W 0对反应混合物所有组分的初始浓度的依赖性。根据这些动力
-
Oxidations by the system “hydrogen peroxide - manganese(IV) complex - acetic acid” — Part II. Hydroperoxidation and hydroxylation of alkanes in acetonitrile作者:Georgiy B Shul'pin、Georg Süss-Fink、John R Lindsay SmithDOI:10.1016/s0040-4020(99)00233-1日期:1999.4ethane, propane, normal butane and isobutane) can be also easily oxidized by the same reagent in acetonitrile solution, the conditions being very mild: low pressure (1–7 bar of the alkane) and low temperature (−22 to +27 °C). Catalyst turnover numbers attain 3100, the yield of oxygenated products is 22% based on the alkane. The yields of oxygenates are higher at low temperatures. The ratio of products高级烷烃(环己烷,正戊烷,正庚烷,甲基丁烷,2-和3-甲基戊烷,3-甲基己烷,顺式和反式十氢化萘)在20°C下在空气中于乙腈中被H 2 O 2氧化(或(IV)锰盐[L 2 Mn 2 O 3 ](PF 6)2存在下(L = 1,4,7-三甲基-1,4-7-三氮杂环壬烷)作为催化剂。反应混合物的必需组分是乙酸。2小时后的营业额达到3300,基于烷烃的氧化产物收率为46%。氧化最初提供相应的烷基氢过氧化物作为主要产物,但是随后这些化合物分解产生相应的酮和醇。反应的区域和键选择性很高:C(1):C(2):C(3):C(4)≈1:40:35:35和1°:2°:3°是1: (15–40):(180–300)。与十氢化萘的两种异构体反应,得到(用PPh 3处理后)在叔位羟基化的醇,如果是顺式,则顺式/反式比为〜2-十氢化萘,如果是反式十氢化萘,则约为30 (即在后者的情况下,反应是立体特异性的)。轻链烷
-
Hydrogen Peroxide Oxygenation of Alkanes Including Methane and Ethane Catalyzed by Iron Complexes in Acetonitrile作者:Georgiy B. Shul'pin、Galina V. Nizova、Yuriy N. Kozlov、Laura Gonzalez Cuervo、Georg Süss-FinkDOI:10.1002/adsc.200303147日期:2004.2This paper describes an investigation of the alkane oxidation with hydrogen peroxide in acetonitrile catalyzed by iron(III) perchlorate (1), iron(III) chloride (2), iron(III) acetate (3) and a binuclear iron(III) complex with 1,4,7-triazacyclononane (4). The corresponding alkyl hydroperoxides are the main products. Nevertheless in the kinetic study of cyclohexane oxidation, the concentrations of oxygenates本文描述了高氯酸铁(III)(1),氯化铁(III)(2),乙酸铁(III)(3)和双核铁(III)络合物催化乙腈中过氧化氢对烷烃的氧化作用的研究。与1,4,7-三氮杂环壬烷(4)。相应的烷基氢过氧化物是主要产物。然而,在环己烷氧化的动力学研究中,在用三苯膦还原反应溶液(将环己基氢过氧化物转化为环己醇)后,测量了含氧化合物(环己酮和环己醇)的浓度。甲烷和乙烷也可以分别用高达30和70的TONs进行氧化。用1添加到氧化溶液中的氯离子激活高氯酸铁衍生物在乙腈中,而水作为添加剂使其失活2在H 2 ö 2分解过程。如果将1或2用作催化剂,则向反应混合物中添加的吡嗪-2-羧酸(PCA)会降低氧化速率,而化合物3和4仅在少量PCA的存在下才具有活性。对氧化动力学和选择性的研究表明,反应的机理是不同的。因此,在1、3 + PCA和4催化的氧化中+ PCA系统的主要氧化物质为羟基自由基,并且假定在2作为催
-
Unusual chemoselective addition of diisopropylzinc to 2,2′-bipyridine-5,5′-dicarbonyl compounds in the 2-position and autoxidative reconversion with carbon–carbon bond cleavage作者:Shigehisa Tanji、Takanori Shibata、Itaru Sato、Kenso SoaiDOI:10.1039/b009474i日期:——Unusual chemoselective addition of diisopropylzinc to the 2-position of 2,2â²-bipyridine-5,5â²-dicarbonyl compounds affords the adducts with a quaternary carbon, and autoxidation of the adducts reconverts them into the initial compounds with carbonâcarbon bond cleavage.
-
Reactions of oxygenated radicals in the gas phase. Part 9.—Self-reactions of isopropylperoxy radicals作者:Leslie T. Cowley、David J. Waddington、Allan WooleyDOI:10.1039/f19827802535日期:——acetaldehyde, formaldehyde, methyl alcohol and cis-2,2′-azopropane. The reaction mechanism has been simulated in detail, and, in conjunction with results obtained earlier for the overall self-reaction of isopropylperoxy radicals, the following rate data have been obtained for the reactions 2(CH3)2CHO2·→(CH3)2CHOH +(CH3)2CO + O2(3a), 2(CH3)2CHO2·→ 2(CH3)2CHO·+ O2(3b)k3b/k3a increases with temperature, from反式-2,2'-偶氮丙烷在333至373 K之间进行光氧化的主要产物是丙酮,异丙醇,异丙基氢过氧化物,乙醛,甲醛,甲醇和顺-2,2'-偶氮丙烷。已详细模拟了反应机理,并结合先前获得的异丙基过氧自由基整体自反应的结果,获得了反应2(CH 3)2 CHO 2 ·→(CH 3)2 CHOH +(CH 3)2 CO + O 2(3 a),2(CH 3)2 CHO 2·→2(CH 3)2 CHO·+ O 2(3 b)k 3 b / k 3 a随着温度的升高而增加,从302 K时的1.39±0.04增加到333 K时的1.83±0.04和373 K时的2.80±0.08。的值阿3一和阿3 b 2.44±0.31×10 7 1.38±0.26×10 9分米3摩尔-1小号-1和Ë 3一和ë 3 b 12.0±1.0和21.3±1.5千焦耳摩尔- 1个 被确定。
表征谱图
-
氢谱1HNMR
-
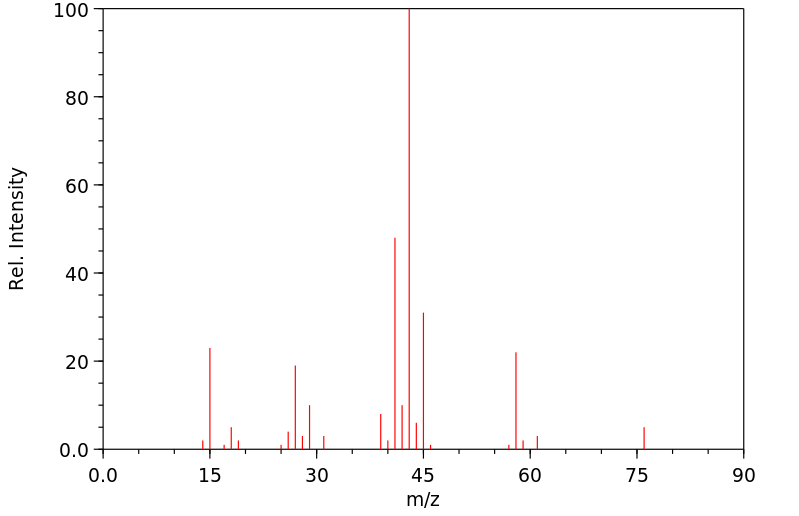
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
过甲酸
过氧化氢,1-(环丙基甲基)-2-甲基丙基
过氧化氢,1,1-二甲基己基
过氧化异丁基甲基甲酮
过氧乙酰亚胺酸
辛基过氧化物
环己基过氧化氢化物
特戊基过氧化氢
氯(氢过氧)甲烷
氫過氧化乙基
氢过氧甲基-环己烷
氢过氧环戊烷
氢过氧基甲烷
氢过氧(甲氧基)甲烷
庚基氢过氧化物
叔己基过氧化氢
叔丁基过氧化氢
二羟基二(羟基-d)锆
二氯(氢过氧)甲烷
乙基-(2-氢过氧基丙-2-基)二氮烯
丙基氢过氧化物
丁基氢过氧化物
8a-氢过氧基-2,3,4,4a,5,6,7,8-八氢-1H-萘
5-辛烯-4-基过氧化氢
5-甲基-2-己基过氧化氢
4-甲氧基-3-(三氟甲基)苯甲基胺
4-氢过氧基-2-戊酮
4-异丙基-1-甲基-2,5-环己二烯-1-基氢过氧化物
4-乙烯基-4-氢过氧基环己烯
4-[(1,1-二甲基乙基)过氧]-1,1,4-三甲基戊基氢过氧化物
3-甲基-3-戊烷基氢过氧化物
3-环己烯基氢过氧化物
3-溴-2-甲基丁烷-2-基过氧化氢
2-过氧化丁酮
2-羟基过氧-2-甲基环己酮
2-癸基过氧化氢
2-环己烯-1-基氢过氧化物
2-氯-1-甲氧基乙基过氧化氢
2-氢过氧基戊烷
2-氢过氧基庚烷
2-氢过氧基丙烷
2-氢过氧基丙-2-基-甲基二氮烯
2-氢过氧-2-甲基丙酸
2-丁基偶氮-2-丙基氢过氧化物
2,5-二甲基正己烷-2,5-二甲羟基过氧化物
2,2-过氧化二氢丙烷
1-甲氧基乙基过氧化氢
1-甲氧基-2-(2-辛氧基-乙氧基)-乙-1-基-过氧化氢
1-甲基环己基过氧化氢
1-甲基-3-(3-丁烯基)环己基过氧化氢