bis-(4,4'-dimethyl-benzhydrylidene)-hydrazine | 5895-68-1
中文名称
——
中文别名
——
英文名称
bis-(4,4'-dimethyl-benzhydrylidene)-hydrazine
英文别名
Bis-(4,4'-dimethyl-benzhydryliden)-hydrazin;4.4'-Dimethyl-diphenylketazin;Di-p-tolyl-ketazin;4,4,4',4'-Tetramethyl-benzophenon-azin;p,p'-Dimethyl-benzophenon-ozin;p,p'-Dimethylbenzophenon-azin;N-[bis(4-methylphenyl)methylideneamino]-1,1-bis(4-methylphenyl)methanimine
CAS
5895-68-1
化学式
C30H28N2
mdl
——
分子量
416.566
InChiKey
ZRGCHWSFOYECMS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:192-194 °C(Solv: ethanol (64-17-5))
-
沸点:528.6±50.0 °C(Predicted)
-
密度:1.02±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):8.5
-
重原子数:32
-
可旋转键数:5
-
环数:4.0
-
sp3杂化的碳原子比例:0.13
-
拓扑面积:24.7
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2928000090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4,4'-Dimethyl-benzophenon-hydrazon 55816-25-6 C15H16N2 224.305 4,4'-二甲基二苯甲酮亚胺 4,4'-dimethylbenzophenone imine 16620-75-0 C15H15N 209.291 —— bis(4-methylphenyl)diazomethane 1143-91-5 C15H14N2 222.29
反应信息
-
作为反应物:参考文献:名称:Mehrotra, K. N.; Joshi, S. C., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1980, vol. 19, # 9, p. 794 - 795摘要:DOI:
-
作为产物:描述:4,4'-Dimethyl-benzophenon-hydrazon 以 乙腈 为溶剂, 反应 0.4h, 以81%的产率得到bis-(4,4'-dimethyl-benzhydrylidene)-hydrazine参考文献:名称:Electrochemical oxidation of benzophenone hydrazones摘要:DOI:10.1021/jo00166a006
文献信息
-
Intermediates in the decomposition of aliphatic diazo-compounds. Part VIII. The mechanism of ether formation from diarylmethylenes and alcohols作者:D. Bethell、A. R. Newall、D. WhittakerDOI:10.1039/j29710000023日期:——The thermal decomposition of diphenyldiazomethane and its 4,4′-dichloro-, 4,4′-dimethoxy-, and 4,4′-dimethyl analogues at 85 °C in acetonitrile containing methyl and t-butyl alcohols (ca. 1M) has been studied. Kinetic studies indicate that the major reaction product, the alkyl diarylmethyl ether, is formed by attack of intermediate diarylmethylene on the alcohol. The relative reactivities of methyl and
-
Selenobenzophenones and Diazoalkanes: Isolation of Tetraarylethylenes by the Reaction of Benzophenone Hydrazones with Diselenium Dibromide作者:Kentaro Okuma、Kazuki Kojima、Kosuke Oyama、Kento Kubo、Kosei ShiojiDOI:10.1002/ejoc.200300510日期:2004.2retrocyclization was observed. The formation of 1,3,4-selendiazolines was independently confirmed by the reaction of benzophenone hydrazones with diselenium dibromide, which afforded tetraarylethylenes in good yields. This method is applicable to the two-step synthesis of tetraarylethylenes from benzophenones. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2004)
-
Carbenerhodium Complexes of the Half-Sandwich-Type: Synthesis, Substitution, and Addition Reactions作者:Helmut Werner、Peter Schwab、Elke Bleuel、Norbert Mahr、Bettina Windmüller、Justin WolfDOI:10.1002/1521-3765(20001215)6:24<4461::aid-chem4461>3.0.co;2-f日期:2000.12.15The substitution products 3 (L=CO) and 4 (L= PMe3) react with an equimolar amount of sulfur or selenium by addition of the chalcogen to the Rh=CPh2 bond to generate the complexes [(eta5-C5H5)Rh(kappa2-ECPh2)(L)] (21-24) with thio- or selenobenzophenone as ligand. Similarly, treatment of 3 with CuCl affords the unusual 1:2 adduct [(eta5-C5H5)(CO)Rh(mu-CPh2)(CuCl)2] (25), which reacts with NaC5H5 to form制备了一系列通式为[(eta5-C5H5)Rh(= CRR')(L)](2a-2i)的碳鎓(I)配合物,其中R = R'=芳基,L = SbiPr3或PR3方平面前体反式-[RhCl(= CRR')(L)2]和Na 的产率很高。三异丙基辛烷衍生物2a的反应。含有不稳定的Rh-Sb键,并带有CO,PMe3和CNR(R = Me,CH2Ph,tBu)导致SbiPr3配体置换并提供取代产物[(eta5- )Rh(= CPh2) (L)](3-7)。相比之下,用CO和CNtBu处理三异丙基膦化合物2c会导致Rh = CPh2键断裂,并通过金属辅助CC产生[(eta5- )Rh(PiPr3)(L)](10,12)。偶合二苯乙烯酮Ph2C = C = O(11)或相应的亚胺Ph2C = C = NtBu(13)。虽然2a的反应 c用C2H4生成[(eta5- )Rh( )
-
Regioselective monoalkylations of the vicinal cis-diol group in mannopyranosides using diaryldiazoalkanes-tin(II) chloride作者:Sigthor Petursson、John M. WebberDOI:10.1016/s0008-6215(82)80006-2日期:1982.5Abstract Highly regioselective monoalkylations of the cis -2,3-diol group in mannopyranosides can be achieved with diaryldiazoalkanes in the presence of catalytic amounts of tin(II) chloride. With diazo(diphenyl)methane ( 1 ), its 4,4′-dimethyl ( 2 ) and 4,4′-dichloro ( 3 ) derivatives, and 9-diazofluorene ( 5 ), methyl 4,6- O -benzylidene- α - d -mannopyranoside gave high yields of the respective
-
Sulphines. Part IV. Reactions of aromatic sulphines with diazoalkanes作者:B. F. Bonini、G. Maccagnani、A. Wagenaar、L. Thijs、B. ZwanenburgDOI:10.1039/p19720002490日期:——The cycloaddition reactions of aromatic sulphines, such as thiobenzophenone S-oxide and thiofluorenone S-oxide, with 2-diazopropane lead to 1,3,4-thiadiazoline 1-oxides in high yields. Diazomethane reacts more sluggishly with sulphines: only with thiofluorenone S-oxide was a cycloadduct obtained. The structure of the cycloadducts has been proven by photochemical extrustion of sulphur monoxide from
表征谱图
-
氢谱1HNMR
-
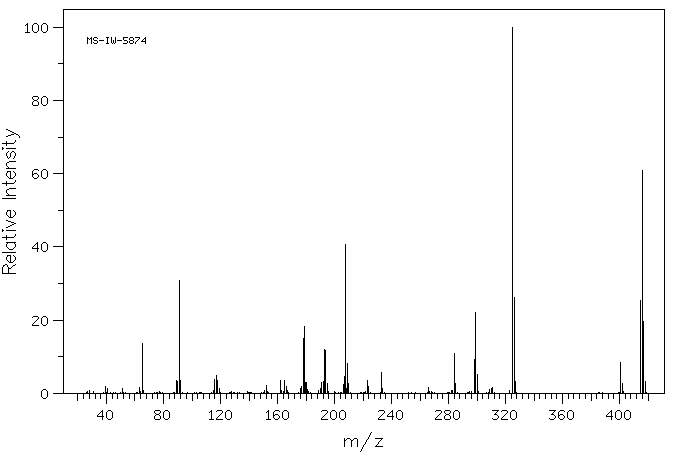
质谱MS
-
碳谱13CNMR
-
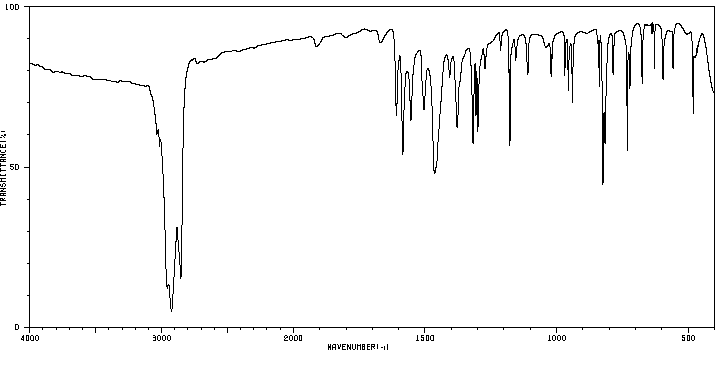
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫