2-氯苯甲酸苄酯 | 7579-40-0
中文名称
2-氯苯甲酸苄酯
中文别名
——
英文名称
benzyl 2-chlorobenzoate
英文别名
2-ClC6H4CO2Bn;2-chloro-benzoic acid benzyl ester;2-Chlor-benzoesaeure-benzylester
CAS
7579-40-0
化学式
C14H11ClO2
mdl
MFCD03784909
分子量
246.693
InChiKey
IJIVXOPMPSQRSF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1893;1904;1867;1883;1900;1900;1914;1929;1944
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:17
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Rapid Chemoselective Deprotection of Benzyl Esters by Nickel Boride摘要:多种酸的苄酯在室温下使用镍硼化物处理甲醇溶液时,可以进行化学选择性裂解,以高产率得到母体羧酸。其他保护功能团,如甲酯、乙酯、叔丁酯、三苯甲酯、苄醚、叔丁醚以及N-苄基酰胺,在这些条件下均不受影响。DOI:10.1055/s-0028-1087982
-
作为产物:参考文献:名称:芳香醛的光化学活化:酰胺,异羟肟酸和酯的合成摘要:已经开发了用于芳族醛活化的便宜,容易且无金属的光化学方案。利用噻吨酮-9-one作为光催化剂,使用廉价的家用灯作为光源,已活化了多种芳香醛,然后通过一锅法将其转化为酰胺,异羟肟酸和酯,产率高至高。该方法的适用性在抗抑郁和社交焦虑药物莫氯贝胺的合成中得到了强调。为了确定该反应的合理机制,已经进行了详细的机理研究。DOI:10.1002/chem.202100655
文献信息
-
Superacid BF<sub>3</sub>–H<sub>2</sub>O promoted benzylation of arenes with benzyl alcohols and acetates initiated by trace water作者:Shuting Zhang、Xiaohui Zhang、Xuege Ling、Chao He、Ruofeng Huang、Jing Pan、Jiaqiang Li、Yan XiongDOI:10.1039/c4ra04059g日期:——
An efficient
in situ prepared superacid BF3–H2O promoted benzylation of arenes using benzyl alcohols and acetates achieves various diarylalkanes. -
Reusable ionic liquid-catalyzed oxidative esterification of carboxylic acids with benzylic hydrocarbons via benzylic Csp<sup>3</sup>–H bond activation under metal-free conditions作者:Fen Mou、Yadong Sun、Weiwei Jin、Yonghong Zhang、Bin Wang、Zhiqing Liu、Lei Guo、Jianbin Huang、Chenjiang LiuDOI:10.1039/c7ra02788e日期:——A metal-free protocol for the direct oxidative esterification of the Csp3–H bond in benzylic hydrocarbons with carboxylic acids using heterocyclic ionic liquid as catalyst has been reported. The catalyst 1-butylpyridinium iodide could be easily recycled and reused for at least four cycles without obvious loss of catalytic activity.
-
Bu4NI-Catalyzed C–C Bond Cleavage and Oxidative Esterification of Allyl Alcohols with Toluene Derivatives作者:Yaoyao Chen、Chengliang Li、Yongmei Cui、Mingming Sun、Xueshun Jia、Jian LiDOI:10.1055/s-0039-1690105日期:2019.10groups for further functionalization and enriches the reactivity profile of allyl alcohol and toluene derivatives. In addition, this protocol represents a new transformation of allyl alcohol involving C–C bond cleavage and C–O bond forming. A novel oxidative esterification of 1-arylprop-2-en-1-ols with toluene derivatives catalyzed by tetrabutylammonium iodide (TBAI) is reported. The optimization of§这些作者同等贡献这项工作。 抽象的 报道了碘化四丁铵(TBAI)催化的1-芳基丙-2-烯-1-醇与甲苯衍生物的新型氧化酯化反应。反应条件的优化表明,每个实验参数,包括催化剂,溶剂和氧化剂,对于目前的氧化功能化都是重要的。这种不含金属的方案具有广泛的底物范围,包括用于进一步官能化的卤素基团,并丰富了烯丙醇和甲苯衍生物的反应活性。此外,该方案代表了烯丙醇的新转变,涉及C–C键断裂和C–O键形成。 报道了碘化四丁铵(TBAI)催化的1-芳基丙-2-烯-1-醇与甲苯衍生物的新型氧化酯化反应。反应条件的优化表明,每个实验参数,包括催化剂,溶剂和氧化剂,对于目前的氧化功能化都是重要的。这种不含金属的方案具有广泛的底物范围,包括用于进一步官能化的卤素基团,并丰富了烯丙醇和甲苯衍生物的反应活性。此外,该方案代表了烯丙醇的新转变,涉及C–C键断裂和C–O键形成。
-
Preparation of Esters and Amides from Carboxylic Acids by Activation with Dialkyl Phosphite-Carbon Tetrachloride Mixture作者:Zsuzsa M. Jászay、Imre Petneházy、László TőokeDOI:10.1080/00397919808004849日期:1998.8Abstract A simple one pot phase transfer catalytic method is described for the synthesis of carboxylic amides and esters from carboxylic acids and amines or alcohols, respectively. For the activation of the carboxylic acids “in situ” generated phosphoric acid diester chlorides were applied.
-
Electrogenerated N-Heterocyclic Carbene in Ionic Liquid: An Insight into the Mechanism of the Oxidative Esterification of Aromatic Aldehydes作者:Gianpiero Forte、Isabella Chiarotto、Achille Inesi、Maria Antonietta Loreto、Marta FerociDOI:10.1002/adsc.201400163日期:2014.5.26An N‐heterocyclic carbene (NHC), generated by cathodic reduction of BMIm BF4, mediates the oxidative esterification of aromatic aldehydes with organic bromides in the corresponding ionic liquid as solvent. The product recovery by simple extractive work‐up with diethyl ether allowed the ionic liquid to be recycled up to 9 times for subsequent electrolyses, with no significant loss in the product yield
表征谱图
-
氢谱1HNMR
-
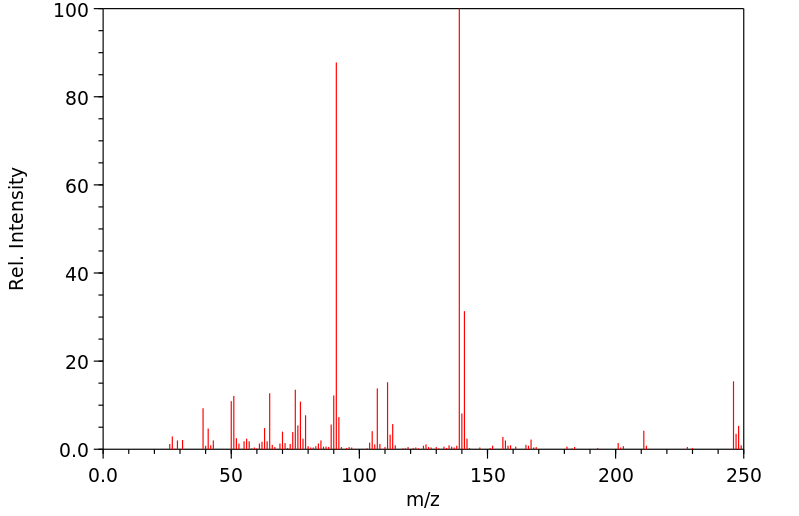
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫