2-溴-7-甲氧基-2,4,6-环庚三烯-1-酮 | 1728-86-5
中文名称
2-溴-7-甲氧基-2,4,6-环庚三烯-1-酮
中文别名
——
英文名称
2-bromo-7-methoxytropone
英文别名
2-bromo-7-methoxycyclohepta-2,4,6-trien-1-one
CAS
1728-86-5
化学式
C8H7BrO2
mdl
——
分子量
215.046
InChiKey
SZPQJNFNDBNPHH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2914700090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基-2,4,6-环庚三烯-1-酮 2-methoxytropone 2161-40-2 C8H8O2 136.15 3-溴-2-羟基-2,4,6-环庚三烯-1-酮 3-bromo-2-hydroxy-2,4,6-cycloheptatrien-1-one 4584-68-3 C7H5BrO2 201.019
反应信息
-
作为反应物:描述:2-溴-7-甲氧基-2,4,6-环庚三烯-1-酮 在 硫酸 lithium perchlorate 、 乙酸酐 作用下, 以 溶剂黄146 为溶剂, 反应 2.0h, 生成 7,7-dimethoxy-2,5-cycloheptadiene-1,4-dione参考文献:名称:Facile Formations ofo- andp-Tropoquinone Mono- and Bisacetals by Anodic Oxidations of 2,3-, 2,5-, 2,7-, and 4,5-Dimethoxytropones摘要:o-和p-萜喹酮的单醚和双醚(四甲氧基环七烯酮)是通过阳极氧化从2,3-、2,5-、2,7-和4,5-二甲氧基萜烯制备的,得到了较高的实用产率。使用铈(IV)盐对二甲氧基萜烯进行氧化,可以得到一系列产物的混合物,即水解产物和/或萜喹酮双醚的甲醇加成物。通过在醋酸和醋酸酐的混合物中与硫酸反应,4,4,7,7和2,2,7,7-四甲氧基环七烯酮生成了具有高位置选择性的Thiele型反应产物。同样,2-溴-7-甲氧基萜烯经过阳极氧化也得到了4,4,7,7-四甲氧基环七烯酮及其2-溴衍生物。反应机制也进行了详细讨论。DOI:10.1246/bcsj.61.3965
-
作为产物:描述:环庚三烯酚酮 在 N-溴代丁二酰亚胺(NBS) 、 18-冠醚-6 、 potassium carbonate 作用下, 以 乙腈 、 苯 为溶剂, 反应 1.0h, 生成 2-溴-7-甲氧基-2,4,6-环庚三烯-1-酮参考文献:名称:假单胞菌 PA14H7:7-羟基托酚酮铁复合物作为抗马铃薯黑胫病病原体 Dickeya 的活性代谢物的鉴定和定量摘要:软腐果杆菌科 (SRP),例如果杆菌属和迪克氏杆菌属,是导致马铃薯等多种作物黑胫病的植物病原菌,影响产量并降低种子生产质量。然而,市场上既没有常规产品也没有生物防治产品来控制这种疾病。在这项研究中,选择假单胞菌 PA14H7(一种从马铃薯根际分离的细菌)作为抗茄病潜伏菌的潜在拮抗剂。为了了解这种拮抗作用的机制,我们设法确定了 PA14H7 产生的主要活性分子。提取 PA14H7 培养物的无细胞上清液 (CFS),并使用 LC-MS、GC-MS 和 NMR 进行分析。我们进一步将提取的 CFS-PA14H7 的抗立枯病生物活性与与铁复合的 7-羟基托酚酮 (7-HT) 的存在相关联。我们第二次合成了该分子,并使用 LC-UV、LC-MS 和 GC-MS 准确测定,在孵育 48 小时后,PA14H7 在其 CFS 中释放了约 9 mg/L 的 7-HT。经评估,在此浓度下,CFS-PA14H7DOI:10.3390/molecules28176207
文献信息
-
[EN] COMPOSITIONS AND METHODS FOR INHIBITING INFLUENZA RNA POLYMERASE PA ENDONUCLEASE<br/>[FR] COMPOSITIONS ET PROCÉDÉS POUR INHIBER L'ENDONUCLÉASE DE LA PA DE L'ARN POLYMÉRASE DE LA GRIPPE申请人:UNIV CALIFORNIA公开号:WO2017156194A1公开(公告)日:2017-09-14There are provided inter alia metalloenzyme inhibitors, such as inhibitors of influenza A RNA dependent RNA polymerase PA subunit endonuclease, and methods of synthesis and use of the same.
-
Monoaryl- and Bisaryldihydroxytropolones as Potent Inhibitors of Inositol Monophosphatase作者:Serge R. Piettre、Catherine André,、Marie-Christine Chanal、Jean-Bernard Ducep、Brigitte Lesur、François Piriou、Pierre Raboisson、Jean-Michel Rondeau、Charles Schelcher、Pascale Zimmermann、Axel J. GanzhornDOI:10.1021/jm9701942日期:1997.12.1The first successful preparation of mono- and disubstituted 3,7-dihydroxytropolone involves a four-step synthetic scheme. Thus, bromination of 3,7-dihydroxytropolone (8) followed by permethylation of the resultant products furnished gram quantities of intermediates 13-18. Single or double Suzuki coupling reactions between these permethylated monobromo- and dibromodihydroxytropolone derivatives and
-
Troponyl-oxamic acid derivatives申请人:Ayerst, McKenna & Harrison Limited公开号:US04125625A1公开(公告)日:1978-11-14Tropone derivatives characterized by having a derivative of oxamic acid at positions 2 and or 5 are disclosed. In addition, the tropone nucleus can be optionally further substituted. The foregoing compounds are useful for preventing or treating allergic conditions in a mammal. Methods for the preparation and use of said compounds are disclosed.
-
The Grignard Reaction of 3-Halotropolone Methyl Ethers作者:Haruki Tsuruta、Toshio MukaiDOI:10.1246/bcsj.41.2489日期:1968.10The Grignard reaction of the methyl ethers (I and III) of 3-bromotropolone and those (II and IV) of 3-chlorotropolone with methylmagnesium iodide or phenylmagnesium bromide were investigated. When I or II was allowed to react with methylmagnesium iodide, 2-methoxyphenyldimethylcarbinol (V) and 2-methoxy-6-isopropylphenol (VI) were obtained. The reaction of I or II with phenylmagnesium bromide afforded
-
Studies on Seven-membered Ring Compounds. XVI. Synthesis of 2-Substituted Cycloheptimidazole Derivatives作者:Hideo Nakao、Genshun SunagawaDOI:10.1248/cpb.13.465日期:——In order to obtain 2-substituted cycloheptimidazole, the reaction of 2-methoxytropone (I) with substituted guanidine and amidine was carried out. Reaction of I with monoalkylguanidine afforded a small amount of 2-alkylaminocycloheptimidazole besides 1-alkyl-2-imino-1, 2-dihydrocycloheptimidazole. Reaction of 2-bromo-7-methoxytropone with monoalkylguanidine afforded 2-alkylamino-4-bromocycloheptimidazole and rearranged product, 2-amino-3-alkyl-4(3H)-quinazolinone. However, reaction of dialkylguanidine with I and 2-bromo-7-methoxytropone afforded only one product, 2-dimethylamino-and 2-dimethylamino-4-bromocycloheptimidazole, respectively. Reaction of aromatic amidine with I or 2-chlorotropone afforded 2-phenyl-and 2-pyridylcycloheptimidazole derivatives. Among cycloheptimidazole derivatives, 2-dimethylaminocycloheptimidazole has been found to undergo easily electrophilic substitution reaction, bromination and nitration.
表征谱图
-
氢谱1HNMR
-
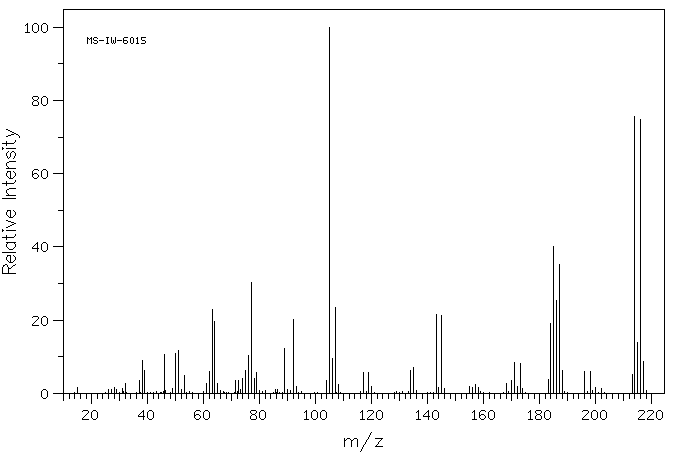
质谱MS
-
碳谱13CNMR
-
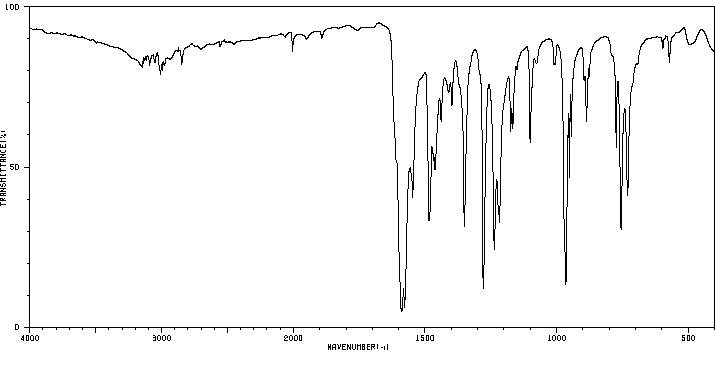
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
脱羰秋水仙碱
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
硫代秋水仙碱
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
环庚三烯酮
环庚三烯酚酮
氨甲酸,(1-乙基戊基)-,甲基酯(9CI)
桧木醇
异秋水仙胺
尼楚酮
对二硫辛酸
双环[4.4.1]十一碳-1(10),2,4,6,8-五烯-11-酮
双环[4.1.0]庚-1,3,5-三烯-7-酮
去乙酰氨基秋水仙碱
原秋水仙碱
十四烷酸,4-(十八烷氧基)-7-羰基-1,3,5-环庚三烯-1-基酯
乙基[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]氨基甲酸酯
三甲基秋水仙素酸
三甲基秋水仙素酸
三(2-羟基-2,4,6-环庚三烯-1-酮)-铟
α-(异丙基)-γ,γ-二甲基环己丙醇
beta-斧松素
[(7S)-7-乙酰氨基-1,3-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-2-基]2-氯乙酸酯
[(7S)-7-乙酰氨基-1,2-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-3-基]2-氯乙酸酯
N-(2-巯基乙基)秋水仙胺
N-脱乙酰基3-去甲基硫代秋水仙碱
N-脱乙酰基,1,2,3,10-脱甲基秋水仙碱
N-甲酰脱乙酰秋水仙碱
N-甲酰基秋水仙胺
N-甲基-秋水仙碱
N-三氟乙酰基-N-甲基-去乙酰基秋水仙碱
N-[(S)-5,6,7,9-四氢-1,2,3,10-四甲氧基-9-氧代苯并[a]庚搭烯-7-基]-2,2,2-三氟乙酰胺
N-[(7S)-4-(羟基甲基)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-10-(丁基氨基)-5,6,7,9-四氢-1,2,3-三甲氧基-9-氧代苯并[a]庚搭烯-7-基]-乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲硫基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲基氨基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]丙酰胺
N-[(7R)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-(乙氧基乙酰基)去乙酰基硫代秋水仙碱
N-(5,6,7,9-四氢-1,2,3-三甲氧基-10-甲硫基-9-氧代苯并[a]庚搭烯-7-基)氨基甲酸乙酯
N-(4-甲酰基-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(10-二甲基氨基-1,2,3-三甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,9-四甲氧基-10-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基)乙酰胺
9H-三苯并[A,C,E][7]环轮烯-9-酮
8H-环庚三烯并[c]异噻唑-8-酮,3-甲基-