2-甲基-3-辛醇 | 26533-34-6
中文名称
2-甲基-3-辛醇
中文别名
——
英文名称
2-methyl-3-octanol
英文别名
2-methyloctan-3-ol
CAS
26533-34-6
化学式
C9H20O
mdl
——
分子量
144.257
InChiKey
DIVBBSLQUDHECU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:6.15°C (estimate)
-
沸点:189 °C
-
密度:0.83
-
保留指数:1092
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905199090
-
储存条件:室温且干燥
SDS
2-甲基-3-辛醇 修改号码:5
模块 1. 化学品
产品名称: 2-Methyl-3-octanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-甲基-3-辛醇
百分比: >98.0%(GC)
CAS编码: 26533-34-6
分子式: C9H20O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
2-甲基-3-辛醇 修改号码:5
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 189 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.83
2-甲基-3-辛醇 修改号码:5
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2-甲基-3-辛醇 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Methyl-3-octanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-甲基-3-辛醇
百分比: >98.0%(GC)
CAS编码: 26533-34-6
分子式: C9H20O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
2-甲基-3-辛醇 修改号码:5
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 189 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.83
2-甲基-3-辛醇 修改号码:5
模块 9. 理化特性
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2-甲基-3-辛醇 修改号码:5
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:机械活化下四乙酸铅-金属卤化物体系氧化脂肪醇摘要:在没有溶剂的情况下,正戊醇、正己醇和正辛醇与 Pb(OAc)4-MHal 系统(M=Li,K;Hal=Cl,Br)的机械化学氧化得到酯。和具有 C8H17OH 和 C9H19OH 组成的仲醇生成酮。DOI:10.1007/bf02494921
-
作为产物:参考文献:名称:Rho-dependent inhibition of the induction of connective tissue growth factor (CTGF) by HMG CoA reductase inhibitors (statins)摘要:假设羟甲基戊二酰辅酶 A(HMG-CoA)还原酶抑制剂(他汀类药物)可能通过干扰 Rho 蛋白的牻牛儿基牻牛儿基化作用来抑制与纤维化相关的因子 CTGF(结缔组织生长因子)的表达。 以人肾成纤维细胞系 TK173 作为体外模型系统,研究他汀类药物介导的肌动蛋白细胞骨架结构以及 CTGF mRNA 表达的调控作用。 将细胞分别与辛伐他汀或洛伐他汀共同孵育,细胞形态及肌动蛋白细胞骨架在时间依赖性和可逆性变化下,约 18 小时时观察到最大效应。 在同一时间段内,他汀类药物降低了 CTGF 的基础表达,并干扰了 CTGF 由溶血磷脂酸(LPA)或转化生长因子 β 所诱导的表达。辛伐他汀和洛伐他汀的作用远强于普伐他汀(IC₅₀ 1–3 μM 与 500 μM 相比)。 当细胞与甲羟戊酸或牻牛儿基牻牛儿基二磷酸(GGPP)共同孵育时,CTGF 表达的抑制作用被逆转,而法尼基二磷酸(FPP)则无效。使用 GTI-286 特异性抑制牻牛儿基牻牛儿基转移酶 I 抑制了 LPA 介导的 CTGF 表达,而法尼基转移酶抑制剂 FTI-276 无效。 辛伐他汀降低了小 GTP 酶 RhoA 在细胞膜的结合。该作用被甲羟戊酸和 GGPP 阻断,但 FPP 无效。 数据支持他汀类药物通过干扰 RhoA 的牻牛儿基牻牛儿基化作用影响 CTGF mRNA 表达的假设,这与先前研究表明 RhoA 是 CTGF 诱导的关键介质一致。 他汀类药物直接干扰 CTGF(与纤维化相关蛋白质)合成,可能是在肾脏疾病中观察到的他汀类药物有益作用的新机制。 *《英国药理学杂志》* (2001), **133**, 1172–1180; **DOI:** [10.1038/sj.bjp.0704173](https://doi.org/10.1038/sj.bjp.0704173)DOI:10.1038/sj.bjp.0704173
文献信息
-
H2O2/HCl system: Oxidation-chlorination of secondary alcohols to α,α′-dichloro ketones作者:Gennady I. Nikishin、Nadezhda I. Kapustina、Lyubov' L. Sokova、Oleg V. Bityukov、Alexander O. Terent'evDOI:10.1016/j.tetlet.2020.152154日期:2020.7The one-pot oxidation-chlorination of secondary alcohols to give α,α′-dichloro ketones in 62–80% yields is reported. The convenient and readily available H2O2/HCl system acts on secondary alcohols (C5-C17) with the formation of α,α′-dichloro ketones. The reaction proceeds with high selectivity under the reported conditions; during this process peroxide formation and the Baeyer-Villiger oxidation reactions
-
A H<sub>2</sub>O<sub>2</sub>/HBr system – several directions but one choice: oxidation–bromination of secondary alcohols into mono- or dibromo ketones作者:Gennady I. Nikishin、Nadezhda I. Kapustina、Liubov L. Sokova、Oleg V. Bityukov、Alexander O. Terent'evDOI:10.1039/c8ra04885a日期:——allows synthesis of α-monobromo ketones and α,α′-dibromo ketones from aliphatic and secondary benzylic alcohols with yields up to 91%. It is possible to selectively direct the process toward the formation of mono- or dibromo ketones by varying the amount of hydrogen peroxide and hydrobromic acid. The convenience of application, simple equipment, multifaceted reactivity, and compliance with green chemistry
-
[EN] PROCESS<br/>[FR] PROCÉDÉ申请人:PHOSPHAGENICS LTD公开号:WO2018112512A1公开(公告)日:2018-06-28An efficient and commercial phosphorylation process of a complex alcohol, such as secondary and tertiary alcohols, with P4O10 at high temperatures, and a product obtained by the process.
-
Enantioselective stereoinversion of sec-alkyl sulfates by an alkylsulfatase from Rhodococcus ruber DSM 44541作者:Mateja Pogorevc、Kurt FaberDOI:10.1016/s0957-4166(02)00362-2日期:2002.7Enantioselective biohydrolysis of sec-alkyl sulfate esters using a bacterial alkylsulfatase from Rhodococcus ruber DSM 44541 proceeded in a stereoselective fashion though inversion of configuration. Thus, from racemic substrates, the corresponding (R)-enantiomers were hydrolyzed selectively to furnish the corresponding sec-alcohol and non-reacted sulfate ester, both of (S)-configuration, which represents
-
Glucosamine-peptide derivatives and their pharmaceutical compositions申请人:Takeda Chemical Industries, Ltd.公开号:EP0002677A1公开(公告)日:1979-07-11Compounds, of the following formula and their physiologically acceptable salts: where: R is hydrogen or a group of the formule R'CO-(wherein R' is an acyclic hydrocarbon group which may be substituted by a cyclic hydrocarbon group -2.3.2,3-dimethoxty-5-methyl-1,4-benzoquinon-6-YI at its ω-position) or a group of the formula They have an immunostimulatory activity.下式化合物及其生理上可接受的盐类: 其中 R 是氢或形式为 R'CO-(wherein R'为无环烃基,可在ω位被环烃基-2.3.2,3-二甲氧基-5-甲基-1,4-苯醌-6-YI 取代)或式中的基团 它们具有免疫刺激活性。
表征谱图
-
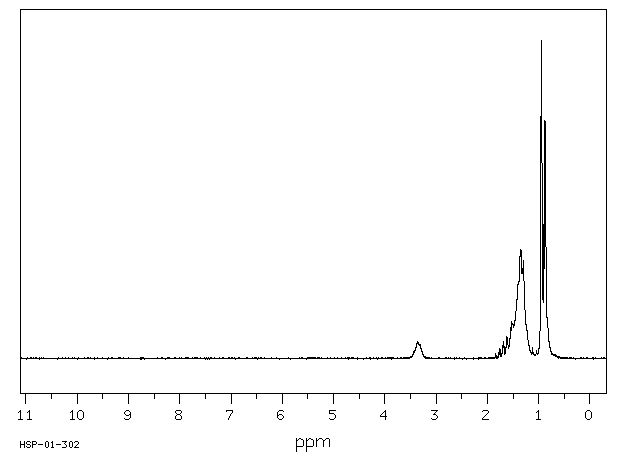
氢谱1HNMR
-
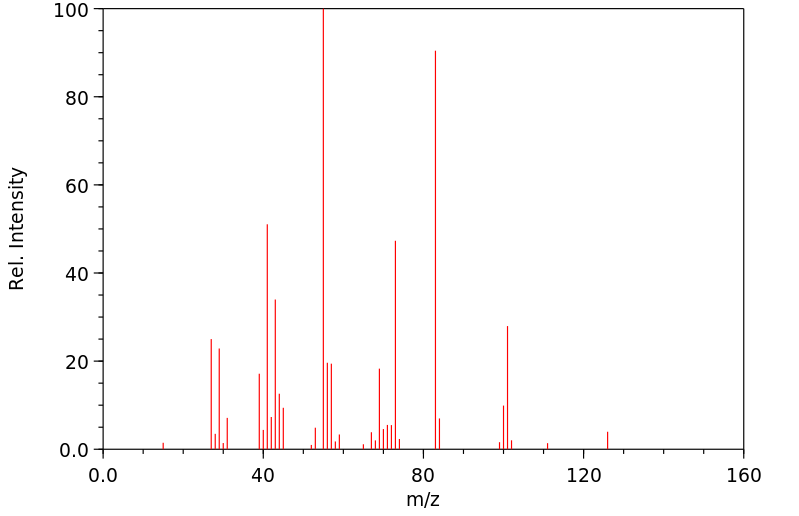
质谱MS
-
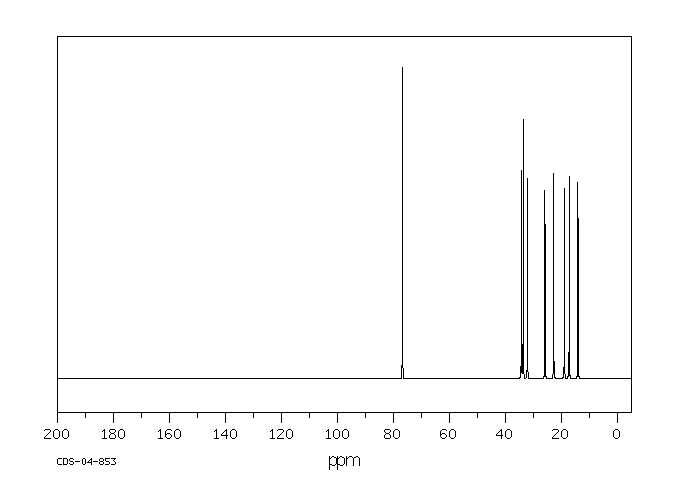
碳谱13CNMR
-
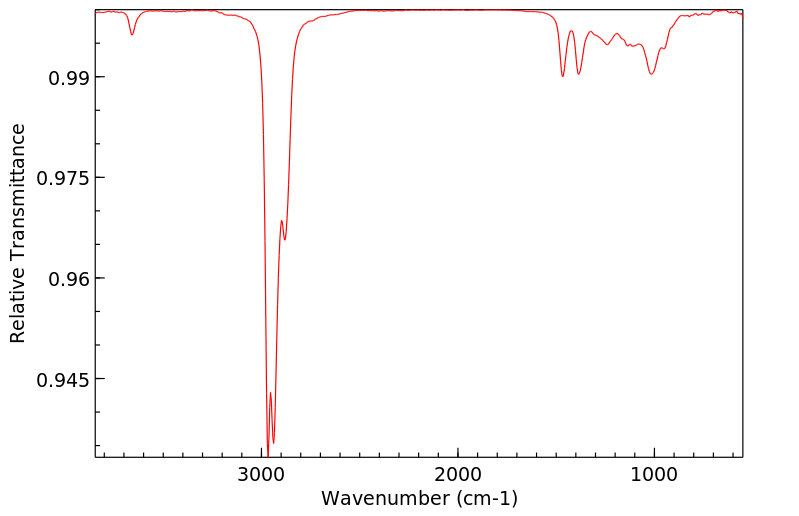
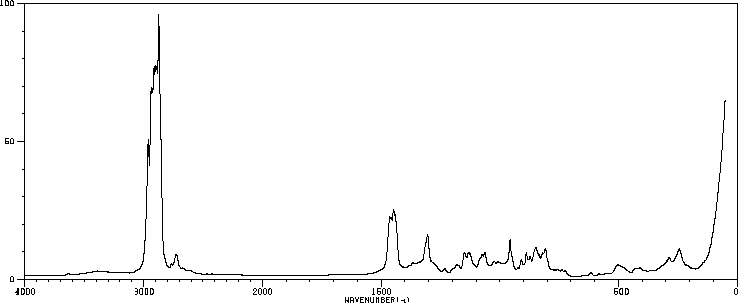
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯