2-甲基-6-硝基苯酚 | 13073-29-5
中文名称
2-甲基-6-硝基苯酚
中文别名
6-硝基邻甲酚;6-硝基邻甲酚 OR 2-甲基-6-硝基苯酚
英文名称
2-methyl-6-nitrophenol
英文别名
6-methyl-2-nitrophenol;2-hydroxy-3-methylnitrobenzene
CAS
13073-29-5
化学式
C7H7NO3
mdl
MFCD00031116
分子量
153.137
InChiKey
AQDKZPFDOWHRDZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:147℃
-
沸点:235.8±20.0℃ (760 Torr)
-
密度:1.320±0.06 g/cm3 (20 ºC 760 Torr)
-
闪点:104.0±10.2℃
-
亨利常数:3.36e-05 atm-m3/mole
-
保留指数:1270
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:66
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2908999090
-
包装等级:III
-
危险类别:6.1
-
危险性防范说明:P501,P273,P270,P264,P280,P302+P352,P337+P313,P305+P351+P338,P362+P364,P332+P313,P301+P312+P330
-
危险品运输编号:2446
-
危险性描述:H301,H315,H319,H412
-
储存条件:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Methyl-6-nitrophenol
Synonyms: 2-Hydroxy-3-nitrotoluene
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Methyl-6-nitrophenol
CAS number: 13073-29-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H7NO3
Molecular weight: 153.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Methyl-6-nitrophenol
Synonyms: 2-Hydroxy-3-nitrotoluene
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Methyl-6-nitrophenol
CAS number: 13073-29-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H7NO3
Molecular weight: 153.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-ethyl-6-nitrophenol 28177-67-5 C8H9NO3 167.164 2-甲基-6-硝基茴香醚 2-methyl-6-nitroanisole 18102-29-9 C8H9NO3 167.164 3-硝基甲苯 1-methyl-3-nitrobenzene 99-08-1 C7H7NO2 137.138 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-硝基水杨醛 3-nitrosalicylic aldehyde 5274-70-4 C7H5NO4 167.121 2-甲基-6-硝基茴香醚 2-methyl-6-nitroanisole 18102-29-9 C8H9NO3 167.164 二硝酚 2-methyl-4,6-dinitrophenol 534-52-1 C7H6N2O5 198.135 —— 2-Butoxy-3-nitrotoluene 911804-98-3 C11H15NO3 209.245 2-甲基-4,6-二硝基苯甲醚 2-methyl-4,6-dinitroanisole 29027-13-2 C8H8N2O5 212.162 —— (2-methyl-6-nitro-phenoxy)-acetic acid —— C9H9NO5 211.174 —— 4-hydroxy-3-methyl-5-nitroacetophenone 375825-57-3 C9H9NO4 195.175 —— acetic acid-(2-methyl-6-nitro-phenyl ester) 70277-91-7 C9H9NO4 195.175 —— 2-methyl-6-nitrophenyl dihydrogen phosphate 1290543-20-2 C7H8NO6P 233.117 6-氨基-2-甲基苯酚 2-amino-6-methylphenol 17672-22-9 C7H9NO 123.155 4-羟基-3-甲基-5-硝基苯磺酸 6-hydroxy-5-nitro-toluene-3-sulfonic acid 85895-88-1 C7H7NO6S 233.202 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Robinson, Journal of the Chemical Society, 1916, vol. 109, p. 1087摘要:DOI:
-
作为产物:描述:参考文献:名称:N-Methyl-2-Chloropyridinium Iodide/NaNO<sub>2</sub>/Wet SiO<sub>2</sub>: Neutral Reagent System for the Nitration of Activated Aromatic Compounds under Very Mild Conditions摘要:在中性、非常温和和环境友好的反应条件下,开发了使用N-甲基-2-氯吡啶碘化物(Mukaiyama试剂)/NaNO2/湿硅胶反应体系对活化芳香化合物进行单硝化的方法。多种结构多样的芳香环在该条件下进行反应,得到相应的硝基芳香化合物,产率中等较高。DOI:10.2174/157017861202150213105836
-
作为试剂:描述:、 邻甲酚 、 硝酸 、 在 乙酸乙酯 、 水 、 碳酸氢钠 、 Sodium sulfate-III 、 silica gel 、 ethyl acetate benzene 、 2-甲基-6-硝基苯酚 作用下, 以 溶剂黄146 为溶剂, 反应 1.0h, 以giving the desired compound, 2-methyl-6-nitrophenol的产率得到2-甲基-6-硝基苯酚参考文献:名称:Acridinium compounds and conjugates thereof摘要:Acridinium化合物的一般化学式为(I),其中A是介入基团,不具有与特定结合物质结合的活性,Z是标记活性基团,具有与特定结合物质结合的活性,R.sup.1是卤素原子,烷基或芳基;R.sup.2,R.sup.3,R.sup.4和R.sup.5分别是氢原子,烷基,芳基,烷氧基,硝基,卤素原子或羰基,Y是计数离子。Acridinium化合物可以与特定结合物质形成共轭物。Acridinium化合物具有高发射效率和稳定性,因此可用作化学发光标记剂。公开号:US05594112A1
文献信息
-
Palladium-catalyzed C–O bond formation: direct synthesis of phenols and aryl/alkyl ethers from activated aryl halides作者:Guoshu Chen、Albert S.C. Chan、Fuk Yee KwongDOI:10.1016/j.tetlet.2006.11.036日期:2007.1palladium-catalyzed carbon–oxygen bond formation is reported. The palladium-tri-tert-butylphosphine complex was found to be effective in converting haloarenes to corresponding substituted phenols. This methodology offers a direct transformation of aryl halides to phenols, as well as the straightforward application to generate a wide variety of diaryl or alkyl/aryl ethers.
-
Highly efficient nitration of phenolic compounds by zirconyl nitrate作者:J. Jon Paul Selvam、V. Suresh、K. Rajesh、S. Ravinder Reddy、Y. VenkateswarluDOI:10.1016/j.tetlet.2006.02.057日期:2006.4Zirconyl nitrate was found to be an excellent reagent in the nitration of phenol and substituted phenols to give nitrated phenols. This procedure works efficiently on most of the examples at room temperature yielding nitro derivatives in fair to good yields with high regioselectivity.
-
A New Method for Nitration of Phenolic Compounds作者:Min Shi、Shi‐Cong CuiDOI:10.1002/adsc.200303111日期:2003.11Phenolic compounds can be nitrated by 65% nitric acid in the presence of catalytic amounts of montmorillonite KSF and bismuth(III) nitrate to give the corresponding nitrated products in good yields in a heterogeneous phase. The co-catalyst of KSF and Bi(NO3)3 can be easily recovered and reused in the next batch of nitration.
-
Calcium Nitrate—A New Nitrating Agent for Nuclear Nitration of Substituted Phenols作者:S. C. Bisarya、S. K. Joshi、A. G. HolkarDOI:10.1080/00397919308018590日期:1993.4Abstract Nitration of selected substituted phenols using Calcium Nitrate as a new nitrating agent is reported, compared with sodium nitrate and the dependance of product selectivity on temperature is demonstrated.
-
Selective Nitration of Aromatic Compounds with Bismuth Subnitrate and Thionyl Chloride作者:Hussni MuathenDOI:10.3390/80700593日期:——Bismuth subnitrate/thionyl chloride have been found to be an efficient combination of reagents for nitration of a wide range of aromatic compounds in dichloromethane. Phenols, in particular, were easily mononitrated and dinitrated with the reagents by controlling the stoichiometry,
表征谱图
-
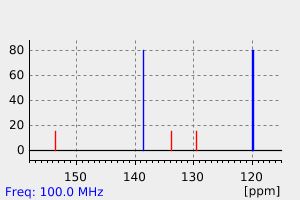
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
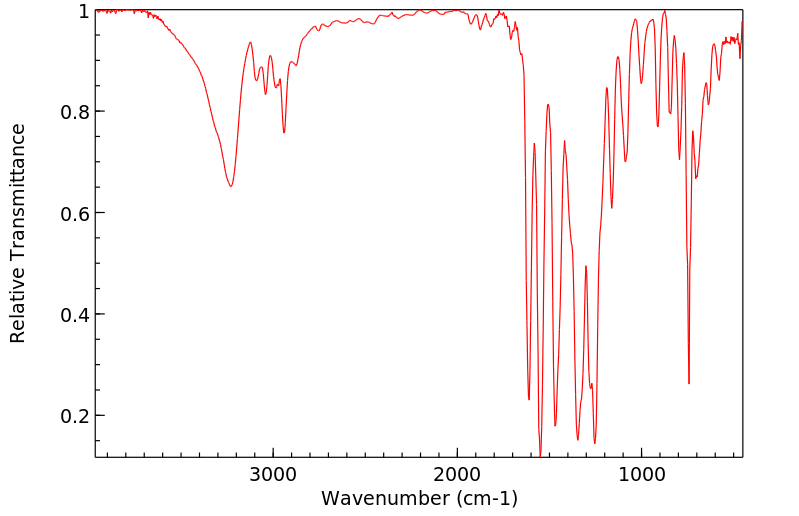
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚