2-硫杂三环(3.3.1.1(3,7))癸烷 | 281-25-4
中文名称
2-硫杂三环(3.3.1.1(3,7))癸烷
中文别名
——
英文名称
2-Thia-adamantan
英文别名
2-Thiaadamantane;2-thiatricyclo[3.3.1.13,7]decane
CAS
281-25-4
化学式
C9H14S
mdl
——
分子量
154.276
InChiKey
NOBCRSKNBOQNEL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1429;1439;1449
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:10
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-Thiaadamantansulfon 23504-97-4 C9H14O2S 186.275
反应信息
-
作为反应物:描述:2-硫杂三环(3.3.1.1(3,7))癸烷 在 Oxone 、 potassium sulfate 、 potassium hydrogensulfate 、 oxone 作用下, 以 甲醇 、 丙酮 为溶剂, 反应 0.17h, 以95%的产率得到2-Thia-tricyclo[3.3.1.13,7]decane 2-oxide参考文献:名称:On the stereochemistry of the oxidation of 5-phenyl-2-thiaadamantane摘要:DOI:10.1021/jo00079a056
-
作为产物:描述:3,7-Diiodobicyclo<3.3.1>nonane 在 sodium sulfide 作用下, 以 乙醇 为溶剂, 反应 10.0h, 以40%的产率得到2-硫杂三环(3.3.1.1(3,7))癸烷参考文献:名称:The Replacement of the Carbonyl Group of Adamantanone by an Oxygen or Sulfur Atom and the One-step Transformation of 2-Methyladamantan-2-ol into 2-Oxa-adamantane; An Efficient New Synthesis of 2-Oxa- and 2-Thiaadamantane摘要:2- 氧杂金刚烷有两种制备方法:以金刚烷酮为原料,通过拜耳-维里格氧化、还原或还原甲基化、环裂碘化和再化学反应四步顺序制备;或以 2-甲基-2-金刚烷醇为原料,通过不分离中间产物的两步反应制备。与此相关,2-thiaadamantane 是由金刚烷酮通过五步反应制备而成,包括拜耳-维里格氧化反应、还原甲基化反应、环裂碘化反应、进一步碘化反应以及 3,7-二碘双环[3.3.1]壬烷与硫化钠的环缩合反应。DOI:10.1055/s-1986-31761
文献信息
-
Sulfur Compounds in Kerosine Boiling Range of Middle East Crudes作者:S. F. Birch、T. V. Cullum、R. A. Dean、R. L. DenyerDOI:10.1021/ie50542a027日期:1955.2
-
SUGINOME HIROSHI; YAMADA SHINJI, SYNTHESIS,(1986) N 9, 741-743作者:SUGINOME HIROSHI、 YAMADA SHINJIDOI:——日期:——
-
The determination of sulfoxide configuration in six-membered rings using NMR spectroscopy and DFT calculations作者:M. Dračínský、R. Pohl、L. Slavětínská、J. Janků、M. BuděšínskýDOI:10.1016/j.tetasy.2011.02.001日期:2011.2Cyclic six-membered ring sulfoxides and sulfones were prepared by a stepwise in situ oxidation of the corresponding sulfides with meta-chloroperbenzoic acid in an NMR tube. The oxidation was followed by NMR spectra and the H-1 and C-13 NMR data were collected. The geometries of all of the compounds were optimized using the DFT B3LYP/6-31G** method and the C-13 and H-1 NMR chemical shifts were calculated for geometry-optimized structures with the DFT B3LYP/6-31++G** method. The calculated C-13 NMR chemical shifts induced by oxidation (Delta delta values) are in very good agreement with the experimental data and can be used to determine the oxidation state of the sulfur atom (-S-, -SO-, -SO2-). The characteristic differences of the induced oxidation chemical shifts of carbon atoms at the alpha- and beta-position to sulfur were successfully used for distinguishing between the diastereoisomeric sulfoxides. (C) 2011 Elsevier Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
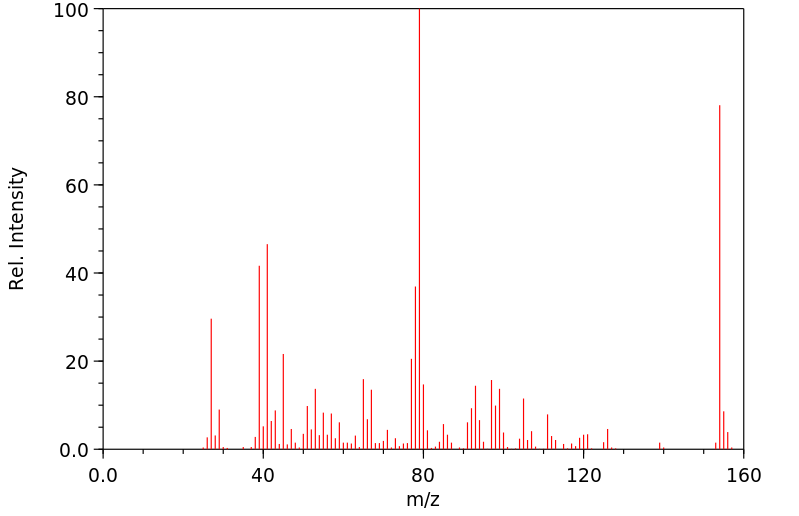
质谱MS
-
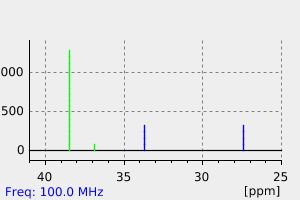
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿普卡林
硫化环戊烷
硫代环己酮
甲基硫酸四氢1-乙基-2-[1,2,3,4--1-(2-羟基乙基)-2,2,4-三甲基-6-喹啉基]苯[cd]吲哚正离子
甲基(1,1-二氧化四氢-2h-噻喃-4-基)醋酸盐
外-3-乙酰基-2-硫杂二环<2.2.2>辛-5-烯
四氢硫代吡喃-4-胺盐酸盐
四氢硫代吡喃-4-羰酰氯
四氢硫代吡喃-4-羧酸甲酯
四氢硫代吡喃-4-甲腈
四氢硫代吡喃-4-基甲醇
四氢硫代吡喃-3-甲醛
四氢噻喃-4-醇
四氢噻喃-4-酮肟
四氢噻喃-4-酮 1,1-二氧化物
四氢噻喃-4-酮
四氢噻喃-4-胺
四氢噻喃-4-肼双盐酸盐
四氢噻喃-4-甲醛
四氢-4H-硫代吡喃-4-酮 1-氧化物
四氢-4-氧代-2H-噻喃-3-甲酸甲酯
四氢-3-甲基-2H-噻喃
四氢-3-氧代-6H-噻喃-2-甲酸甲酯
四氢-2H-硫代吡喃-4-醇 1,1-二氧化物
四氢-2H-硫代吡喃-4-羧醛 1,1-二氧化物
四氢-2H-硫代吡喃-4-甲醇 1,1-二氧化物
四氢-2H-硫代吡喃-4-乙醛
四氢-2H-噻喃-4-羧酸甲酯1,1-二氧化物
四氢-2H-噻喃-4-甲酰肼
四氢-2H-噻喃-4-甲腈1,1-二氧化
四氢-2H-噻喃-3-醇1,1-二氧化物
四氢-2H-噻喃-3-羧酸1,1-二氧化物
四氢-(9ci)-2H-硫代吡喃-4-羧酸
噻-4-基甲胺
叔-丁基[(1S,2R)-1-苯甲基-2-羟基-3-[异丁基[(4-硝基苯基)磺酰]氨基]丙基]氨基甲酸酯
二氢-5,5-二甲基-2H-硫基吡喃-3(4H)-酮-1,1-二氧化物
二氢-2H-硫代吡喃-3(4h)-酮
二氢-2H-硫代吡喃-3(4H)-酮-1,1-二氧化物
乙酸四氢-2H-噻喃-2-基酯
三环己基乙基硼酸钠
n-[四氢-2H-硫代吡喃-4-基]氨基甲酸-1,1-二甲基乙酯
N-甲基四氢-2H-硫代吡喃-4-胺盐酸盐
N-甲基四氢-2H-噻喃-4-胺盐酸盐
N-甲基(四氢硫代吡喃-4-基)甲基胺
9-硫杂二环[3.3.1]壬烷-2,6-二酮
9-硫杂二环[3.3.1]壬烷
8-硫杂二环[3.2.1]辛烷-3-酮
8-硫杂-2,3-二氮杂螺[4.5]癸烷
8-乙烯基-7-硫杂-二环[4.2.0]辛烷
7-硫杂-2-氮杂螺[3.5]壬烷半草酸酯