2-碘丙烯 | 4375-96-6
中文名称
2-碘丙烯
中文别名
——
英文名称
2-iodopropene
英文别名
2-iodo-propene;isopropenyl iodide;2-Jod-propen;β-Jod-propylen;Allylenhydrojodid;2-Jod-prop-1-en;2-iodoprop-1-ene
CAS
4375-96-6
化学式
C3H5I
mdl
MFCD00040004
分子量
167.977
InChiKey
KWAHADSKPFGJQF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-99.29°C (estimate)
-
沸点:87.11°C (estimate)
-
密度:1.7856
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903399090
SDS
反应信息
-
作为反应物:参考文献:名称:Reactions of Gaseous, Halogenated Propene Radical Cations with Ammonia: A Study of the Mechanism by Fourier Transform Ion Cyclotron Resonance摘要:DOI:10.1002/(sici)1521-3765(19980904)4:9<1799::aid-chem1799>3.0.co;2-7
-
作为产物:描述:参考文献:名称:Ssemenow, Zeitschrift fur Chemie, 1865, p. 725摘要:DOI:
文献信息
-
An Efficient Route to Benzene and Phenol Derivatives via Ring-Closing Olefin Metathesis作者:Kazuhiro Yoshida、Tsuneo Imamoto、Shingo Horiuchi、Noriyuki Iwadate、Fumihiro KawagoeDOI:10.1055/s-2007-982548日期:2007.6Without the formation of inseparable regioisomers, various substituted phenol derivatives 2 and benzene derivatives 4 were prepared through RCM-tautomerization and RCM-dehydration protocols. A new synthetic route to precursors 1 and 3 enabled efficient access to these aromatic compounds.
-
Selective grignard-type carbonyl addition of alkenyl halides mediated by chromium(II) chloride作者:Kazuhiko Takai、Keizo Kimura、Tooru Kuroda、Tamejiro Hiyama、Hitosi NozakiDOI:10.1016/s0040-4039(00)88417-8日期:1983.1Alkenyl (or aryl) iodide (or bromide) is readily reduced with CrCl2 is N,N-dimethylformamide at 25°C to gice the corresponding organochromium species which adds selectively to an aldehyde moiety without affecting the coexisting ketone or cyano group of the substrate.
-
Gold-Catalyzed Highly Regioselective Oxidation of C−C Triple Bonds without Acid Additives: Propargyl Moieties as Masked α,β-Unsaturated Carbonyls作者:Biao Lu、Chaoqun Li、Liming ZhangDOI:10.1021/ja1072614日期:2010.10.13Gold-catalyzed intermolecular oxidations of internal alkynes have been achieved with high regioselectivities using 8-alkylquinoline N-oxides as oxidants and in the absence of acid additives. Synthetically versatile α,β-unsaturated carbonyls are obtained in good to excellent yields and with excellent E-selectivities. A range of functional groups such as THP, MOMO, N(3), OTBS, and N-Boc are tolerated
-
Gold‐Catalyzed Double Cycloisomerization of 1‐Ene‐4,10‐diynyl Esters to Bicyclo[6.3.0]undeca‐2,4,9‐trienyl Esters作者:Mitch Mathiew、Javey Khiapeng Tan、Philip Wai Hong ChanDOI:10.1002/anie.201809376日期:2018.10.22trienyl esters efficiently from gold(I)‐catalyzed Rautenstrauch rearrangement/1,5‐hydride shift/8‐endo‐dig cyclization of 1‐ene‐4,10‐diynyl esters is described. The suggested double cycloisomerization mechanism delineates the first example of an unactivated all‐carbon tethered 1,7‐enyne, either preformed or formed in situ, which undergoes an 8‐endo‐dig cyclization pathway to give a cyclooctane motif
-
Silver Triflate-Catalyzed Cyclopropenation of Internal Alkynes with Donor-/Acceptor-Substituted Diazo Compounds作者:John F. Briones、Huw M. L. DaviesDOI:10.1021/ol201503j日期:2011.8.5Silver triflate was found to be an efficient catalyst for the cyclopropenation of internal alkynes using donor-/acceptor-substituted diazo compounds as carbenoid precursors. Highly substituted cyclopropenes, which cannot be synthesized directly via rhodium(II)-catalyzed carbenoid chemistry, can now be readily accessed.
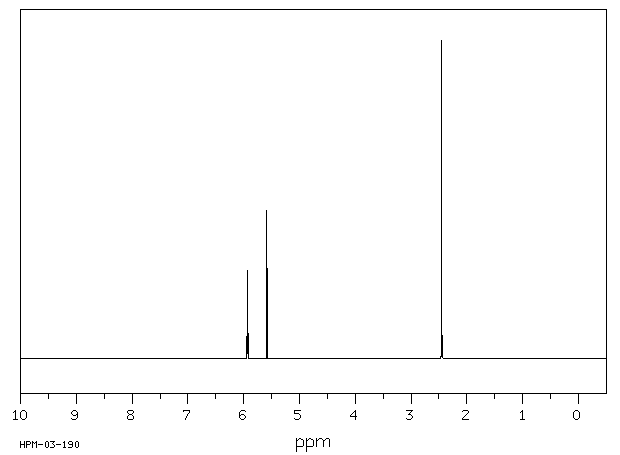
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-3-甲基-1,2,3,4-四氯-1-丁烯
顺式-1-溴-1-丙烯
顺式-1-氯-1-丁烯
顺式-1,3-二氯丙烯
顺式-1,2-二碘乙烯
顺式-1,2-二溴乙烯
顺式-1,2-二氟-1-氯乙烯
顺-氯丹
顺-九氯
顺-九氯
顺-1-溴-2-乙氧基乙烯
顺-1,2-二氯乙烯
顺-1,2,4-三氯-3-甲基-2-丁烯
顺,顺-1,2,3,4-四氯-1,3-丁二烯
除螨灵
锗烷,(1-溴-1,2-丙二烯基)三甲基-
锌,氯(三氟乙烯基)-
铜(1+),1,1,2-三氟乙烯
苯甲酸,4-[(1E)-2-[[(4-氯苯基)甲基]磺酰]乙烯基]-
苯并烯氟菌唑中间体
艾日布林-2碘
聚(乙烯-氯代三氟乙烯)
碳化镁碘化物
碘化乙烯
硫丹醇
硅烷,二氯(2-氯乙烯基)甲基-
硅烷,[2-(碘亚甲基)己基]三甲基-,(Z)-
甲碘乙烯
甲氧基全氟丁烷-反式-1,2-二氯乙烯1:1共沸物
甲基烯丙基溴化镁
甲基全氟-1-甲基-2-丙烯基醚
甲基全氟-1-丁基-1-丙烯基醚
甲基全氟-1-丁基-1-丙烯基醚
环丙烷,1,1-二氯-2-(3,3-二氯-2-甲基-2-丙烯基)-2,3,3-三甲基-
环丙烯,1,2-二氟-
特比萘芬杂质
溴西克林
溴甲基烯酮
溴环辛四烯
溴氯丙烯
溴代三氟代乙烯
溴亚甲基环己烷
溴乙烯
溴三碘乙烯
氰尿酰氟
氯磺酸三氟乙烯基酯
氯化聚乙烯
氯乙烯与异丁基乙烯醚共聚物
氯乙烯与三氯乙烯聚合物
氯乙烯-d3