2-羟基芴 | 2443-58-5
中文名称
2-羟基芴
中文别名
2-芴醇
英文名称
2-hydroxyfluorene
英文别名
9H-fluoren-2-ol;2-Hydroxyfluoren;2-fluorenol
CAS
2443-58-5
化学式
C13H10O
mdl
MFCD00010778
分子量
182.222
InChiKey
ZDOIAPGLORMKTR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:167-169 °C (lit.)
-
沸点:266.3 °C
-
密度:0.9982 g/cm3
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
物理描述:Solid
-
保留指数:1941;277.3
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:14
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.076
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
储存条件:请将药品存放在密闭、避光、通风干燥的地方保存。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 2-Hydroxyfluorene
CAS-No. : 2443-58-5
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Skin irritation (Category 2)
Eye irritation (Category 2)
Specific target organ toxicity - single exposure (Category 3)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Irritating to eyes, respiratory system and skin.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Precautionary statement(s)
Avoid breathing dust.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R36/37/38 Irritating to eyes, respiratory system and skin.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S37/39 Wear suitable gloves and eye/face protection.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : 2-Fluorenol
Formula : C13H10O
Molecular Weight : 182,22 g/mol
Component Concentration
9H-Fluoren-2-ol
CAS-No. 2443-58-5 -
EC-No. 219-478-9
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end use(s)
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: powder
Colour: tan
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 167 - 169 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Causes respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. Causes skin irritation.
Eyes Causes serious eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine Pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基-9H-芴 2-methoxy-9H-fluorene 2523-46-8 C14H12O 196.249 芴 9H-fluorene 86-73-7 C13H10 166.222 2-羟基-9-芴 2-hydroxyfluoren-9-one 6949-73-1 C13H8O2 196.205 —— 2-hydroxy-9-fluorenol 106593-45-7 C13H10O2 198.221 2-氟芴 2-fluoro-9H-fluorene 343-43-1 C13H9F 184.213 2-溴芴 2-bromo-9H-fluorene 1133-80-8 C13H9Br 245.118 2-碘芴 2-iodo-9H-fluorene 2523-42-4 C13H9I 292.119 2-氨基芴 2-aminofluorene 153-78-6 C13H11N 181.237 芴-2-硼酸 2-fluoreneboronic acid 480424-61-1 C13H11BO2 210.04 2-乙酰芴 1-(9H-fluoren-2-yl)ethanone 781-73-7 C15H12O 208.26 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基-9H-芴 2-methoxy-9H-fluorene 2523-46-8 C14H12O 196.249 —— 2-Hydroxy-3-methyl-fluoren 5092-99-9 C14H12O 196.249 烯丙基-芴-2-基醚 allyl-fluoren-2-yl ether 103394-93-0 C16H14O 222.287 —— 2-acetoxyfluorene 2443-56-3 C15H12O2 224.259 —— 3-amino-2-hydroxy fluorene 107623-30-3 C13H11NO 197.236 2-羟基-9-芴 2-hydroxyfluoren-9-one 6949-73-1 C13H8O2 196.205 甲磺酸芴-2-基酯 methanesulfonic acid fluoren-2-yl ester 100621-99-6 C14H12O3S 260.313 —— 7-methoxy-9H-fluorene-2-carboxylic acid 107942-89-2 C15H12O3 240.258 —— 2-Acetyl-7-methoxyfluoren 5018-73-5 C16H14O2 238.286 —— 2-(9H-fluoren-2-yloxy)-5-methylhexanoic acid 27747-75-7 C20H22O3 310.393 —— 2-(9H-fluoren-2-yloxy)-5,5-dimethylhexanoic acid 27747-74-6 C21H24O3 324.42 2-乙酰基-7-(辛氧基)芴 2-acetyl-7-(octyloxy)fluorene 102887-26-3 C23H28O2 336.474 —— ethyl 2-(9H-fluoren-2-yloxy)heptanoate 27747-53-1 C22H26O3 338.447 —— diethyl 2-(9H-fluoren-2-yloxy)-2-methylpropanedioate 27747-94-0 C21H22O5 354.403 2-羟基-芴-1-羧酸 2-hydroxy-fluorene-1-carboxylic acid 106782-30-3 C14H10O3 226.232 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Garcia de Varela, 1955, vol. 20, p. 7,11摘要:DOI:
-
作为产物:参考文献:名称:Biogenic synthesis of Fe 2 O 3 @SiO 2 nanoparticles for ipso -hydroxylation of boronic acid in water摘要:Here, biogenic synthesis of Fe2O3@5iO(2) nanoparticles using fruit extract of Zanthoxylum rhetsa is reported. The SiO2 nanoparticles was synthesized using paddy straw which is a byproduct obtained in cultivation of rice. The composite was characterised by spectroscopic method like XRD, SEM, TEM and EDX analysis. The ipso-hydroxylation reactions were carried out with excellent yield within a moderate time period with mild reaction condition in all cases. Therefore, this approach may be considered as simple, easy, cheap and greener, environment friendly protocol for ipso-hydroxylation of arylboronic acids at 50 degrees C temperature. (C) 2017 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2017.09.075
文献信息
-
QUENCHER申请人:Wako Pure Chemical Industries, Ltd.公开号:US20170342031A1公开(公告)日:2017-11-30A quencher is disclosed having a compound represented by the following general formula (1): wherein R 5 each independently represent a halogen atom, an alkyl group, an alkoxy group, an alkylthio group, an amino group having a substituent or not having a substituent, a hydroxy group, an aryl group, an aryloxy group, or an arylalkyl group; R 6 represents a group having a polymerizable unsaturated group, a hydroxy group, or the like; Y 1 represents an oxygen atom, or the like; An − represents an anion; Ar 1 represents a specific ring structure; * and ** represent binding positions; Ar 2 represents a benzene ring, a naphthalene ring, or an anthracene ring; n 1 represents a specific integer; and the following structure (1-10) in the general formula (1) is an asymmetric structure; (wherein R 5 , Y 1 , Ar 1 , Ar 2 , n 1 , * and ** are the same as described above.).
-
Synthesis of Phenols: Organophotoredox/Nickel Dual Catalytic Hydroxylation of Aryl Halides with Water作者:Liu Yang、Zhiyan Huang、Gang Li、Wei Zhang、Rui Cao、Chao Wang、Jianliang Xiao、Dong XueDOI:10.1002/anie.201710698日期:2018.2.12A highly effective hydroxylation reaction of aryl halides with water under synergistic organophotoredox and nickel catalysis is reported. The OH group of the resulting phenols originates from water, following deprotonation facilitated by an intramolecular base group on the ligand. Significantly, aryl bromides as well as less reactive aryl chlorides served as effective substrates to afford phenols with
-
Orthoamides, LVI [1]. A New Method of Wide Scope for the Preparation of Aryl Formates作者:Georg Ziegler、Willi KantlehnerDOI:10.1515/znb-2001-1112日期:2001.11.1
Aryl formates 4a-u, 6 , 8 , 10, 12, 14, 16, 18, 20, 22, 24, 26 are prepared by formylation of hydroxyarenes 3a-u, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25 with N,N-diformylacetamide (1) or triformamide (2), respectively, in fairly good yields. The reactions can be catalyzed by sodium diformamide or praseodymium(III) triflate. The thiolformate 28 was obtained analogously from 1-thionaphthol (27).
-
Design, synthesis, and testing of potential antisickling agents. 4. Structure-activity relationships of benzyloxy and phenoxy acids作者:D. J. Abraham、P. E. Kennedy、A. S. Mehanna、D. C. Patwa、F. L. WilliamsDOI:10.1021/jm00374a006日期:1984.8small molecules, benzyloxy and phenoxy acids, as potent inhibitors of hemoglobin S (HbS) gelation. Structural modifications with a large number of each class confirm our earlier work that the highest activity is observed with compounds that contain dihalogenated aromatic rings with attached polar side chains. We have also found a halogenated aromatic malonic acid derivative to be quite active. Compounds
-
TETRADENTATE AND OCTAHEDRAL METAL COMPLEXES CONTAINING NAPHTHYRIDINOCARBAZOLE AND ITS ANALOGUES申请人:Arizona Board of Regents on behalf of Arizona State University公开号:US20160359125A1公开(公告)日:2016-12-08Tetradentate and octahedral metal complexes suitable for use as phosphorescent or delayed fluorescent and phosphorescent emitters in display and lighting applications.
表征谱图
-
氢谱1HNMR
-
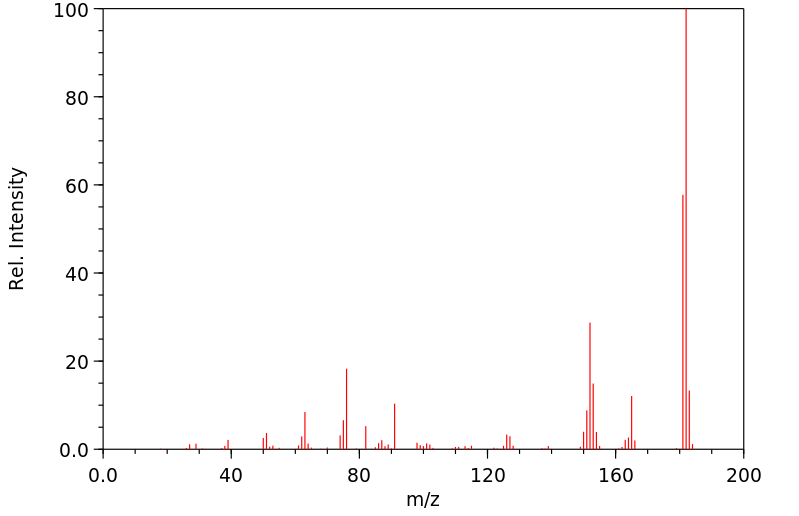
质谱MS
-
碳谱13CNMR
-
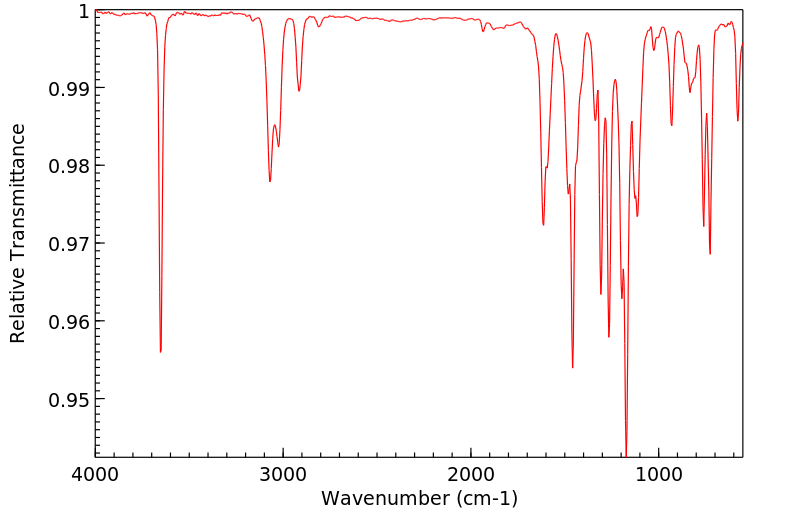
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-2-N-Fmoc-氨基甲基吡咯烷盐酸盐
(2S,4S)-Fmoc-4-三氟甲基吡咯烷-2-羧酸
黎芦碱
鳥胺酸
魏因勒卜链接剂
雷迪帕韦二丙酮合物
雷迪帕韦中间体6
雷迪帕韦
雷迪帕维中间体
雷迪帕维中间体
雷尼托林
锰(2+)二{[乙酰基(9H-芴-2-基)氨基]氧烷负离子}
醋酸丁酸纤维素
达托霉素杂质
赖氨酸杂质4
试剂9,9-Dioctyl-9H-fluoren-2-amine
螺[环戊烷-1,9'-芴]
螺[环庚烷-1,9'-芴]
螺[环己烷-1,9'-芴]
螺[3.3]庚烷-2,6-二-(2',2'',7',7''-四碘螺芴)
螺-(金刚烷-2,9'-芴)
螺(环己烷-1,9'-芴)-3-酮
藜芦托素
荧蒽 反式-2,3-二氢二醇
草甘膦-FMOC
英地卡胺
苯芴醇杂质A
苯甲酸-(芴-9-基-苯基-甲基酯)
苯甲酸-(9-苯基-芴-9-基酯)
苯并[b]芴铯盐
苯并[a]芴酮
苯基芴胺
苯基(9-苯基-9-芴基)甲醇
苯(甲)醛,9H-芴-9-亚基腙
苯(甲)醛,4-羟基-3-甲氧基-,(3-甲基-9H-茚并[2,1-c]吡啶-9-亚基)腙
芴甲氧羰酰胺
芴甲氧羰酰基高苯丙氨酸
芴甲氧羰酰基肌氨酸
芴甲氧羰酰基环己基甘氨酸
芴甲氧羰酰基正亮氨酸
芴甲氧羰酰基D-环己基甘氨酸
芴甲氧羰酰基D-Β环己基丙氨酸
芴甲氧羰酰基-O-三苯甲基丝氨酸
芴甲氧羰酰基-D-正亮氨酸
芴甲氧羰酰基-6-氨基己酸
芴甲氧羰基-高丝氨酸内酯
芴甲氧羰基-缬氨酸-1-13C
芴甲氧羰基-叔丁基二甲基硅-D-丝氨酸
芴甲氧羰基-beta-赖氨酰酸(叔丁氧羰基)
芴甲氧羰基-S-叔丁基-L-半胱氨酸五氟苯基脂