3,3'-二甲基吡啶胺 | 1656-94-6
中文名称
3,3'-二甲基吡啶胺
中文别名
双(3-吡啶基甲基)胺;3,3'-双(吡啶甲基)胺;α,α'-亚胺二(3-甲基吡啶);3,3-二甲基吡啶胺;3,3"-二甲基吡啶胺
英文名称
bis(pyridin-3-ylmethyl)amine
英文别名
di(3-pyridylmethyl)amine;di(3-picolyl)amine;N,N'-bis(3-pyridylmethyl)amine;dpma;Bis((3-pyridyl)methyl)amine;1-pyridin-3-yl-N-(pyridin-3-ylmethyl)methanamine
CAS
1656-94-6
化学式
C12H13N3
mdl
MFCD00038043
分子量
199.255
InChiKey
FEBQXMFOLRVSGC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:168 °C(Press: 0.55 Torr)
-
密度:1,12 g/cm3
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:15
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:37.8
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2933399090
-
储存条件:存储条件:室温下,保存于惰性气体中。
SDS
Revision number: 5
Bis(3-pyridylmethyl)amine
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Bis(3-pyridylmethyl)amine
Product name:
5
Revision number:
Section 2. HAZARDS IDENTIFICATION
GHS classification
Not classified
PHYSICAL HAZARDS
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Category 2A
Serious eye damage/eye irritation
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Warning
Signal word
Causes skin irritation
Hazard statements
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
Substance/mixture:
Bis(3-pyridylmethyl)amine
Components:
>97.0%(GC)
Percent:
1656-94-6
CAS Number:
3,3'-Dipicolylamine , α,α'-Iminodi(3-picoline)
Synonyms:
C12H13N3
Chemical Formula:
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Get medical advice/attention if you feel unwell.
Bis(3-pyridylmethyl)amine
Section 4. FIRST AID MEASURES
Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
Skin contact:
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Rinse cautiously with water for several minutes. Remove contact lenses, if present
Eye contact:
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
from the chemical:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
When extinguishing fire, be sure to wear personal protective equipment.
Special protective
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Comply with laws.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Bis(3-pyridylmethyl)amine
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Clear
Form:
Very pale yellow - Yellow
Colour:
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
No data available
Boiling point/range:
No data available
Flash point:
Flammability or explosive
limits:
No data available
Lower:
No data available
Upper:
1.12
Relative density:
Solubility(ies):
No data available
[Water]
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
ivn-mus LD50:250 mg/kg
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
No data available
Germ cell mutagenicity:
Carcinogenicity:
No data available
IARC =
No data available
NTP =
No data available
Reproductive toxicity:
UT4397000
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
No data available
Crustacea:
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
No data available
Log Pow:
No data available
Soil adsorption (Koc):
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Bis(3-pyridylmethyl)amine
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Bis(3-pyridylmethyl)amine
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Bis(3-pyridylmethyl)amine
Product name:
5
Revision number:
Section 2. HAZARDS IDENTIFICATION
GHS classification
Not classified
PHYSICAL HAZARDS
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Category 2A
Serious eye damage/eye irritation
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Warning
Signal word
Causes skin irritation
Hazard statements
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
Substance/mixture:
Bis(3-pyridylmethyl)amine
Components:
>97.0%(GC)
Percent:
1656-94-6
CAS Number:
3,3'-Dipicolylamine , α,α'-Iminodi(3-picoline)
Synonyms:
C12H13N3
Chemical Formula:
Section 4. FIRST AID MEASURES
Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Inhalation:
Get medical advice/attention if you feel unwell.
Bis(3-pyridylmethyl)amine
Section 4. FIRST AID MEASURES
Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
Skin contact:
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Rinse cautiously with water for several minutes. Remove contact lenses, if present
Eye contact:
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
from the chemical:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
When extinguishing fire, be sure to wear personal protective equipment.
Special protective
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Comply with laws.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Bis(3-pyridylmethyl)amine
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Clear
Form:
Very pale yellow - Yellow
Colour:
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
No data available
Boiling point/range:
No data available
Flash point:
Flammability or explosive
limits:
No data available
Lower:
No data available
Upper:
1.12
Relative density:
Solubility(ies):
No data available
[Water]
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
ivn-mus LD50:250 mg/kg
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
No data available
Germ cell mutagenicity:
Carcinogenicity:
No data available
IARC =
No data available
NTP =
No data available
Reproductive toxicity:
UT4397000
RTECS Number:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
No data available
Crustacea:
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
No data available
Log Pow:
No data available
Soil adsorption (Koc):
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Bis(3-pyridylmethyl)amine
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:From 1D Helix to 0D Loop: Nitrite Anion Induced Structural Transformation Associated with Unexpected N-Nitrosation of Amine Ligand摘要:An infinite Ag(I) coordination 4(1)-helical chain, [Ag(Hdpma)](NO3)2 center dot H2O (1), was synthesized by the self-assembly of AgNO3 and di(3-pyridylmethyl)amine (dpma). Helix 1 is 5-fold interweaved and has a topological diamondoid-like net that is extended by ligand-unsupported helix-to-helix argentophilic interactions. Two identical diamondoid-like nets with opposite chiralities interpenetrate to form the whole 3D framework as a meso compound. Typical anion-exchange reactions cause a remarkable single-crystal-to-single-crystal (SCSC) structural transformation from the 1D helix 1 to the 0D molecular loop [Ag(dpma-NO)(NO2)](2) (2) (induced by the nitrite anion, NO2-) and a 1D molecular ladder [Ag(dpma)(H2O)](NO3) (induced by the fluoride anion, F-). Molecular loop 2 is an N-nitroso compound. This work is the first to present observations of nitrite-dominated in situ N-nitrosation of an amine ligand which accompanies SCSC structural transformation via an anion-exchange reaction.DOI:10.1021/ic500306y
-
作为产物:参考文献:名称:负载型铜催化剂将伯胺非均相催化自缩合为仲胺†摘要:廉价的负载型铜催化剂Cu / Al 2 O 3可通过还原氢氧化物前体Cu(OH)x / Al 2 O 3轻松制备,从而有效地促进伯胺向对称取代的仲胺的自缩合。各种结构不同的伯胺,包括苄胺, 苦瓜胺,并且脂肪族胺衍生物可以选择性地转化为相应的仲胺,且产率中等至优异,而在Ar或H 2的1 atm中没有任何助催化剂,例如碱和稳定的配体。与Ar中的反应相比,H 2中的反应对所需仲胺的选择性更高。H 2的作用是促进N-烷基亚胺的氢化和稳定活性Cu(0)物种。另外,在Cu / Al 2 O 3的存在下,可以通过伯胺与醇的N-烷基化和醛的还原胺化来有效地合成不对称取代的仲胺。观察到的催化确实是非均质的,并且回收的Cu / Al2 O 3催化剂可再用于自冷凝,而不会显着降低其催化性能。已经提出了涉及伯胺的脱氢和缩合为N-烷基亚胺然后氢化的反应机理,即所谓的“借用氢途径”。DOI:10.1039/c3cy00405h
-
作为试剂:描述:cadmium(II) nitrate tetrhydrate 、 N,N-二甲基乙酰胺 、 2,6-萘二羧酸 在 3,3'-二甲基吡啶胺 作用下, 以 甲醇 为溶剂, 反应 72.0h, 以84%的产率得到[Cd3(2,6-naphthalenedicarboxylate)3(N,N'-dimethylacetamide)4]参考文献:名称:Synthesis, characterization, and dye capture of a 3D Cd(II)–carboxylate pcu network摘要:The hydro(solvo)thermal reaction of Cd(NO3)(2)center dot 4H(2)O and 2,6-naphthalenedicarboxylic acid (2,6-H(2)ndc) together with di(3-pyridylmethyl)amine, being as structure -direct reagent, in N,N'-dimethylacetamide (DMAc)-MeOH at 120 degrees C afforded a cadmium-carboxylate network, which is formulated as [Cd-3(2,6-ndc)(3)(DMAc)(4)] (1) by single -crystal X-ray diffraction analysis. The extended architecture of 1 suits a three-dimensional (3D) six -connected slanted 4(12).6(3)-pcu framework based on trinuclear Cd-3(O2C)(6)(DMAc)(4) clusters being symmetry-related by a 2-fold rotation axis. Dye adsorption explorations for methyl blue, methyl orange (MO), methylene blue (MB), rhodamine B (RB), and methyl yellow (MY) dyes clearly indicate that 1 has exhibited dye capture behavior for methyl blue from an aqueous solution, and can selectively bind methyl blue from a MO-methyl blue mixture. In addition, the thermal stability and photoluminescence properties of the crystalline material have also been studied. (C) 2016 Elsevier Ltd. All rights reserved.DOI:10.1016/j.poly.2016.11.025
文献信息
-
Selective cross-coupling of amines by alumina-supported palladium nanocluster catalysts作者:Ken-ichi Shimizu、Katsuya Shimura、Keiichiro Ohshima、Masazumi Tamura、Atsushi SatsumaDOI:10.1039/c1gc15835j日期:——Al2O3-supported Pd nanoclusters with an average particle size of 1.8 nm act as a reusable catalyst for the selective cross-coupling of amines. The reaction is a structure-sensitive reaction, demanding coordinatively unsaturated Pd atoms on a metallic nanocluster. The support also affects the activity, an amphoteric oxide (Al2O3) is most effective.
-
Microwave-assisted synthesis of primary amine HX salts from halides and 7 M ammonia in methanol作者:Mark G. Saulnier、Kurt Zimmermann、Charles P. Struzynski、Xiaopeng Sang、Upender Velaparthi、Mark Wittman、David B. FrennessonDOI:10.1016/j.tetlet.2003.10.146日期:2004.1on a variety of alkyl halides, as well as mesylates and tosylates. Benzylamines are obtained from benzyl halides without significant amounts of the secondary amine side products that result without microwave heating. Direct isolation of even highly volatile primary amines as their hydrogen halide salts makes the method ideal for use in parallel synthesis.
-
ORGANIC SALTS AND METHOD FOR PRODUCING CHIRAL ORGANIC COMPOUNDS申请人:List Benjamin公开号:US20090030216A1公开(公告)日:2009-01-29The invention relates to a method for producing chiral organic compounds by asymmetric catalysis, using ionic catalysts comprising a chiral catalyst anion. The claimed method is suitable for reactions which are carried out over cationic intermediate stages, such as iminium ions or acyl pyridinium ions. The invention enables the production of chiral compounds with high ee values, that until now could only be obtained by means of costly purification methods.
-
Metal-Free Catalyst for the Chemoselective Methylation of Amines Using Carbon Dioxide as a Carbon Source作者:Shoubhik Das、Felix D. Bobbink、Gabor Laurenczy、Paul J. DysonDOI:10.1002/anie.201407689日期:2014.11.17N‐methylation of amines is an important step in the synthesis of many pharmaceuticals and has been widely applied in the preparation of other key intermediates and chemicals. Therefore, the development of efficient methylation methods has attracted considerable attention. In this respect, carbon dioxide is an attractive C1 building block because it is an abundant, renewable, and nontoxic carbon source
-
New pyrrolidine and pyrroline derivatives of fullerenes: from the synthesis to the use in light-converting systems作者:P. A. Troshin、A. S. Peregudov、S. I. Troyanov、R. N. LyubovskayaDOI:10.1007/s11172-008-0126-4日期:2008.5pyrroline derivatives of fullerenes C60 and C70 were synthesized and characterized. The proposed procedures afford the reaction products in yields twice as high (80–85%) as those attained by the classical Prato reaction. The reactions proceed with virtually complete regio- (in the case of C70) and stereoselectivity to afford only cis-2′,5′-disubstituted and trans-1′,2′,5′-trisubstituted pyrrolidinofullerenes作者对由吡啶甲胺和苄胺衍生物生成的偶氮甲碱和腈叶立德的 [2+3] 环加成反应生成富勒烯的研究结果进行了系统化,并考虑了新的实验数据。催化剂和微波辐射促进叶立德的形成以及它们与富勒烯的加入首次被成功使用。合成并表征了大量新的富勒烯 C60 和 C70 的吡咯烷和吡咯啉衍生物。所提出的程序提供的反应产物的产率是经典普拉托反应的两倍(80-85%)。该反应以几乎完全的区域-(在 C70 的情况下)和立体选择性进行,仅提供顺式-2',5'-二取代和反式-1',2',5'-三取代的吡咯烷富勒烯。吡啶基取代的吡咯烷富勒烯与金属卟啉和酞菁反应形成自序配位配合物。后者是自然光合天线系统的类似物,因为这些复合物在暴露于光线时会发生光致电荷分离。
表征谱图
-
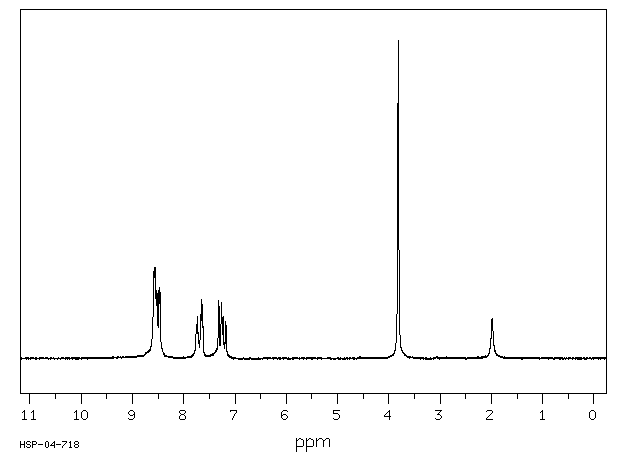
氢谱1HNMR
-
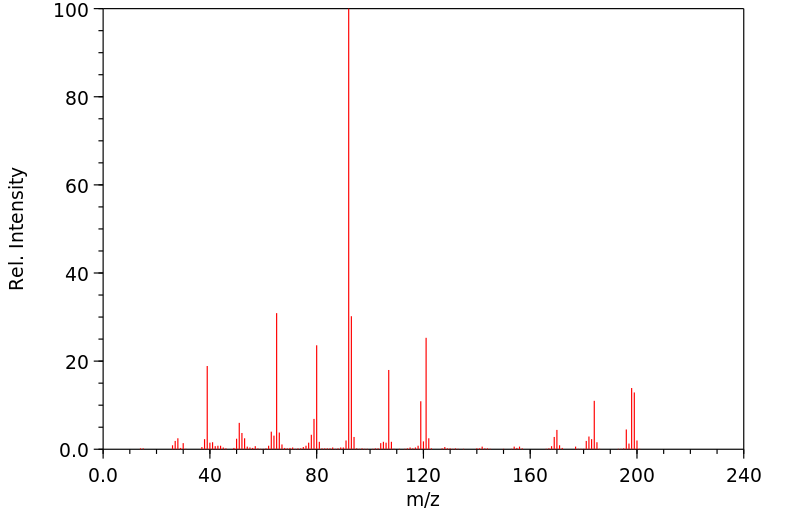
质谱MS
-
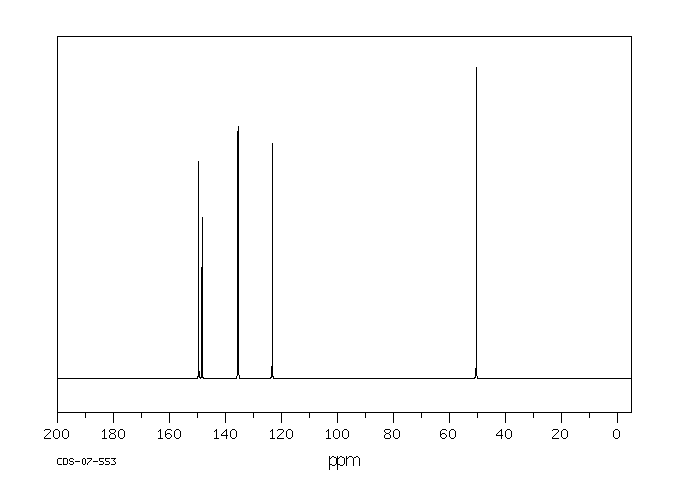
碳谱13CNMR
-
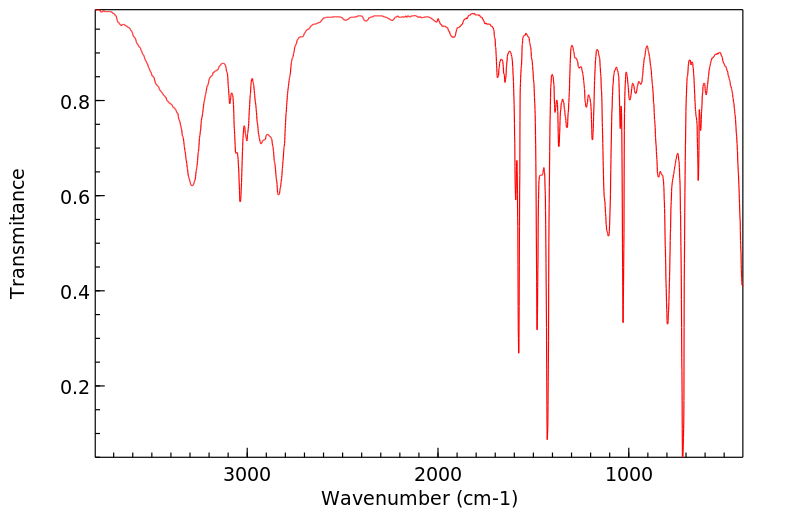
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷