3,5-二溴苄基溴 | 56908-88-4
中文名称
3,5-二溴苄基溴
中文别名
3,5-二溴溴苄;α,3,5-三溴甲苯;3,5-二溴苄溴
英文名称
3,5-dibromobenzyl bromide
英文别名
1,3-dibromo-5-(bromomethyl)benzene
CAS
56908-88-4
化学式
C7H5Br3
mdl
——
分子量
328.829
InChiKey
PWTFRUXTAFBWBW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:93-97 °C
-
沸点:276.85°C (rough estimate)
-
密度:2.1881 (estimate)
-
溶解度:溶于甲苯
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,没有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:8
-
安全说明:S26,S36/37/39,S45
-
危险品运输编号:1759
-
海关编码:2903999090
-
危险类别:8
-
危险品标志:F,C
-
危险类别码:R34,R11
-
包装等级:III
-
危险性防范说明:P210,P240,P264,P280,P301+P330+P331,P303+P361+P353,P304+P340,P305+P351+P338,P310,P363,P370+P378,P403,P501
-
危险性描述:H228,H314
-
储存条件:请将贮藏器保持密封,并储存在阴凉、干燥的地方。同时,确保工作环境具有良好的通风或排气设施。
SDS
| Name: | 3 5-Dibromobenzyl bromide Material Safety Data Sheet |
| Synonym: | alpha,3,5-Tribromobenzyl bromide; 1,3-Dibromo-5-(bromomethyl)benzen |
| CAS: | 56908-88-4 |
Synonym:alpha,3,5-Tribromobenzyl bromide; 1,3-Dibromo-5-(bromomethyl)benzen
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 56908-88-4 | 3,5-Dibromobenzyl bromide | 97% | unlisted |
Risk Phrases: 11 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Highly flammable. Causes burns.
Potential Health Effects
Eye:
Causes eye burns. Lachrymator (substance which increases the flow of tears).
Skin:
Causes skin burns.
Ingestion:
Causes gastrointestinal tract burns.
Inhalation:
Causes chemical burns to the respiratory tract.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Flammable solid.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Remove all sources of ignition. Use a spark-proof tool.
Section 7 - HANDLING and STORAGE
Handling:
Use spark-proof tools and explosion proof equipment. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage:
Keep away from sources of ignition. Store in a cool, dry place.
Store in a tightly closed container. Flammables-area. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 56908-88-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white crystalline solid
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 88 - 90 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H5Br3
Molecular Weight: 329
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
No information found.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Water, oxidizing agents, bases, alcohols, amines, steel.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen bromide, bromine.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 56908-88-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,5-Dibromobenzyl bromide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing Group: II
IMO
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: F C
Risk Phrases:
R 11 Highly flammable.
R 34 Causes burns.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 56908-88-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 56908-88-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 56908-88-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-二溴甲苯 3,5-dibromotoluene 1611-92-3 C7H6Br2 249.933 3,5-二溴苯甲醛 3,5-dibromo benzaldehyde 56990-02-4 C7H4Br2O 263.916 3,5-二溴苯甲醇 (3,5-dibromophenyl)methanol 145691-59-4 C7H6Br2O 265.932 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,5-二溴苯甲醛 3,5-dibromo benzaldehyde 56990-02-4 C7H4Br2O 263.916 3,5-二溴苯甲醇 (3,5-dibromophenyl)methanol 145691-59-4 C7H6Br2O 265.932 —— (3,5-dibromophenyl)methanamine 771580-86-0 C7H7Br2N 264.947 —— 3,5-dibromobenzyl mercuric bromide 97578-06-8 C7H5Br3Hg 529.419 —— 1-(3,5-dibromophenyl)-N-methylmethanamine 216755-73-6 C8H9Br2N 278.974 3,5-二溴苯乙腈 2-(3,5-dibromophenyl)acetonitrile 188347-48-0 C8H5Br2N 274.942
反应信息
-
作为反应物:描述:参考文献:名称:溶液 DFWM χ(3) 含四配位或超配位硅的聚[(亚芳基)甲硅烷基]和聚[(亚芳基)(乙炔基)甲硅烷基]的非线性光学性质摘要:在氯仿溶液中研究了一系列共轭聚[(亚芳基)(亚乙炔基)甲硅烷基]的三阶光学非线性,以及一系列互补的聚[(亚芳基)甲硅烷基]统计共聚物,没有乙炔基团。使用简并四波混频技术和 Q 开关 Nd:YAG 激光振荡器,波长为 1064 nm,脉冲持续时间为 ca。6纳秒。该聚合物含有多种亚芳基,包括咔唑和蒽。χ(3) 磁化率和解的热非线性的电子和核贡献是分开的。对于浓度为 50 gl-1 的溶液,发现一些聚 [(亚芳基)(亚乙炔基)亚硅烷基] 的 χ(3) 磁化率高达 ∣Re χ(3) ∣=0.95×10-10 esu。结果表明,在硅上存在提供五配位的单个 8-(二甲氨基)萘基配体对 χ(3) 特性具有有益的影响。比较聚[(亚芳基)亚硅烷基]s 与聚[(亚芳基)(亚乙炔基)亚硅基]s 的结果表明,前一种情况下乙炔基团的缺失通常对 χ( 3) 属性。非线性响应的数量级的确认已通过 Z 扫描测量得到证实DOI:10.1039/b003046p
-
作为产物:描述:3,5-二溴苯甲醇 在 N-溴代丁二酰亚胺(NBS) 、 双(三甲基硅烷基)氨基钾 、 1,3,4-三苯基-4,5-二氢-1H-1,2,4-三氮唑-5-亚基 作用下, 以 二氯甲烷 为溶剂, 反应 6.0h, 以174 mg的产率得到3,5-二溴苄基溴参考文献:名称:N-杂环卡宾对Appel型反应的促进作用†摘要:N-杂环卡宾(NHC)已广泛用作有机催化和有机金属化学中的一类通用催化剂和配体。但是,只有少数几种合成应用程序充当试剂。在这里,我们证明了NHC可用作化学计量的氧化还原试剂,用于醇的Appel型卤化反应。这种新的反应性揭示了一个新鲜有趣的方面,并丰富了未开发区域中NHC的化学性质。还研究了使用NHC-氧化物衍生物在催化水平上进行此化学转化的潜力。DOI:10.1039/c9cc02132a
文献信息
-
Design, synthesis, and functional assessment of Cmpd-15 derivatives as negative allosteric modulators for the β2-adrenergic receptor作者:Kaicheng Meng、Paul Shim、Qingtin Wang、Shuai Zhao、Ting Gu、Alem W. Kahsai、Seungkirl Ahn、Xin ChenDOI:10.1016/j.bmc.2018.03.023日期:2018.5The β2-adrenergic receptor (β2AR), a G protein-coupled receptor, is an important therapeutic target. We recently described Cmpd-15, the first small molecule negative allosteric modulator (NAM) for the β2AR. Herein we report in details the design, synthesis and structure-activity relationships (SAR) of seven Cmpd-15 derivatives. Furthermore, we provide in a dose-response paradigm, the details of the所述β 2 -肾上腺素能受体(β 2 AR),G蛋白偶联受体,是一个重要的治疗目标。我们最近描述CMPD-15,第一小分子负变构调节剂(NAM)为β 2 AR。本文中,我们详细报告了7种CMPD-15衍生物的设计,合成和构效关系(SAR)。此外,我们提供一种在剂量-响应范例,它们的衍生物的效果的细节在调节激动剂诱导的β 2在放射性配体竞争结合试验中,AR活性(G蛋白介导的cAMP产生和β-arrestin募集到受体)以及正构激动剂的结合亲和力。我们的研究结果表明,一些修改,包括在除去甲酰胺基的第-formAMido苯丙氨酸区域和溴在元-bromobenzyl甲基苯甲酰胺区域引起CMPD-15的功能活性显着降低。这些SAR结果提供了宝贵的见解NAM CMPD-15的作用机制以及为更有效和选择性调节剂的未来发展,为β的基础2基于CMPD-15的化学支架AR。
-
Pyrimidine-thioalkyl and alkylether compounds申请人:PHARMACIA & UPJOHN COMPANY公开号:EP1247804A1公开(公告)日:2002-10-09The subject invention relates to pyrimidine-thioalkyl and alkylether compounds of Formula I and pyrimidine-thioalkyl and alkylethers of Formula IA, namely the compounds of Formula I where R4 is selected from the group consisting of -H or -NR15R16 where R15 is -H and R16 is -H, C1-C6 alkyl, -NH2 or R15 and R16 taken together with the -N form 1-pyrrolidino, 1-morpholino or 1-piperidino; and R6 is selected from the group consisting of -H, or halo (preferably -Cl); with the overall provisio that R4 and R6 are not both -H; The compounds of Formula IA are useful in the treatment of individuals who are HIV positive.
-
[EN] PHOTOAFFINITY PROBES<br/>[FR] SONDES DE PHOTOAFFINITÉ申请人:PROMEGA CORP公开号:WO2020191339A1公开(公告)日:2020-09-24Provided herein are compositions and methods for photoaffinity labeling of molecular targets. In particular, probes that specifically interact with cellular targets based on their affinity and are then covalently linked to the cellular target via a photoreactive group (PRG) on the probe.本文件提供了用于光亲和标记分子靶点的组合物和方法。特别是,基于它们的亲和力与细胞靶点特异性相互作用的探针,然后通过探针上的光反应组(PRG)与细胞靶点共价连接。
-
Chemical synthesis and evaluation of 17α-alkylated derivatives of estradiol as inhibitors of steroid sulfatase作者:Diane Fournier、Donald PoirierDOI:10.1016/j.ejmech.2011.06.027日期:2011.9Steroid sulfatase (STS) controls the levels of 3-hydroxysteroids available from circulating steroid sulfates in several normal and malignant tissues. This and the known involvement of active estrogens and androgens in diseases such as breast and prostate cancers thus make STS an interesting therapeutic target. Here we describe the chemical synthesis and characterization of an extended series of 17α-derivatives
-
一种2-取代的一枝蒿酮酸甲酯衍生物及其制 备方法和用途申请人:中国科学院新疆理化技术研究所公开号:CN107089913B公开(公告)日:2020-10-09本发明涉及2‑取代的一枝蒿酮酸甲酯衍生物及其制备方法和用途,该类衍生物是由一枝蒿酮酸和硫酸二甲酯合成一枝蒿酮酸甲酯后,和不同取代的卤代烃反应,在强碱二异丙基氨基锂的作用下合成而得,并对所合成的衍生物3a‑3q进行了初步的体外抗A型(H3N2,H1N1)和B型流感病毒活性测试。实验结果表明,大部分化合物表现出了抗甲型流感病毒(H1N1)的活性,尤其是化合物3b(IC50=0.82μg·mL‑1),3c(IC50=0.82μg·mL‑1),3i(IC50=1.92μg·mL‑1)和化合物3j(IC50=1.43μg·mL‑1)具有比阳性对照物(达菲和利巴韦林)更强的抗病毒活性;对于 亚型,只有3e,3l,3q表现出了活性;对抗B型流感,3i,3j,3o表现出了活性。该方法反应条件温和,实验步骤简捷。
表征谱图
-
氢谱1HNMR
-
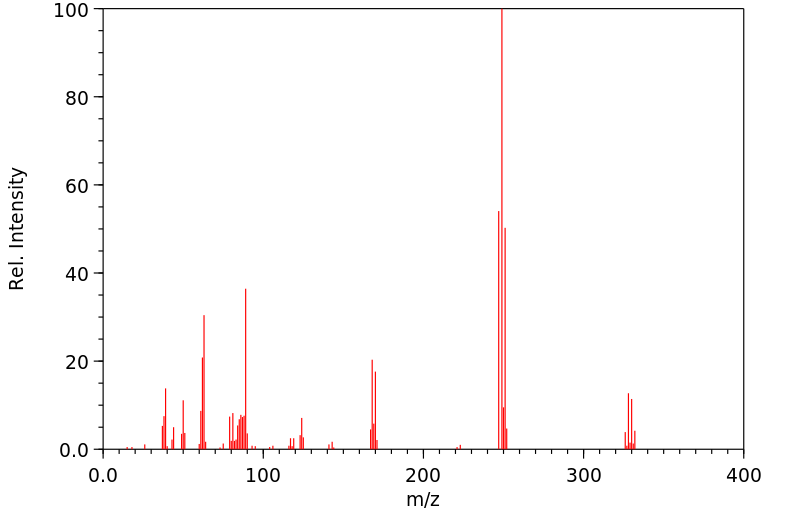
质谱MS
-
碳谱13CNMR
-
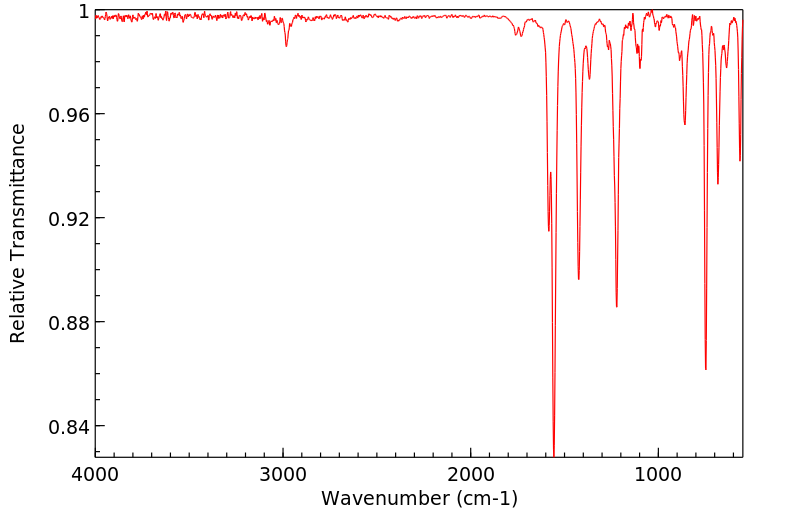
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫