(E)-3-(3-(4 (Dimethylamino)Phenyl)Acrylo-yl)-4-Hydroxy-2H-Chromen-2-One | 57339-82-9
中文名称
——
中文别名
——
英文名称
(E)-3-(3-(4 (Dimethylamino)Phenyl)Acrylo-yl)-4-Hydroxy-2H-Chromen-2-One
英文别名
(E)-3-(3-(4-(Dimethylamino)phenyl)acryloyl)-4-hydroxy-2H-chromen-2-one;3-[3-[4-(dimethylamino)phenyl]prop-2-enoyl]-4-hydroxychromen-2-one
CAS
57339-82-9
化学式
C20H17NO4
mdl
MFCD10001312
分子量
335.359
InChiKey
RGEZPZWWPVZCIT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:222-225 °C
-
沸点:563.5±50.0 °C(Predicted)
-
密度:1.351±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:25
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:66.8
-
氢给体数:1
-
氢受体数:5
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-乙酰基-2-羟基苯并吡喃-4-酮 3-acetyl-4-hydroxychromen-2-one 2555-37-5 C11H8O4 204.182
反应信息
-
作为反应物:描述:(E)-3-(3-(4 (Dimethylamino)Phenyl)Acrylo-yl)-4-Hydroxy-2H-Chromen-2-One 在 盐酸 、 sodium acetate 作用下, 以 乙醇 、 水 为溶剂, 生成 3-(5-(4-(dimethylamino)phenyl)-4,5-dihydro-1-(4-phenylthiazol-2-yl)-1H-pyrazol-3-yl)-4-hydroxy-2H-chromen-2-one参考文献:名称:靶向VEGFR-2激酶并诱导细胞周期停滞和凋亡的新型噻唑基吡唑基香豆素衍生物的合成,抗癌作用和分子模型。摘要:合成了新的噻唑基吡唑基香豆素衍生物,并在体外针对五种不同的人类细胞系(包括乳腺癌MCF-7,肺A549,前列腺PC3,肝HepG2和正常黑素细胞HFB4)测试了其抗癌潜力。乳腺癌显示出对化合物7a,8c,9b,9c和9d的更高敏感性,与参考药物阿霉素相比,IC50值在5.41至10.75μM之间(IC50 = 6.73μM)。另外,对正常细胞HFB4没有显示出明显的毒性。此外,对人乳腺癌MCF-7细胞系中VEGFR-2抑制作用的体外研究显示,该细胞毒性化合物显示出化合物7a,8c,9b,9c和9d在低微摩尔浓度下是有效的抑制剂(IC50 = 0.034-0.582 μM)与参考药物索拉非尼(IC50 = 0.019μM)进行比较。进行了一些理论和实验研究,以揭示控制乳腺癌转移的分子机制。由于其对MCF-7的显着细胞毒活性和对VEGFR-2的显着抑制作用,对有前途的化合物9d评估了细胞周期进DOI:10.1016/j.bioorg.2018.12.040
-
作为产物:描述:4-羟基香豆素 在 哌啶 、 三氯氧磷 作用下, 以 氯仿 为溶剂, 生成 (E)-3-(3-(4 (Dimethylamino)Phenyl)Acrylo-yl)-4-Hydroxy-2H-Chromen-2-One参考文献:名称:Synthesis of Some Coumarinyl Chalcones and their Antiproliferative Activity Against Breast Cancer Cell Lines摘要:研究人员合成了一系列香豆素基查尔酮衍生物,并评估了它们对三种不同乳腺癌细胞系(MDA-MB231、MDA-MB468、MCF7)和一种非癌症乳腺上皮细胞系(184B5)的抗增殖活性。香豆素基衍生物对乳腺癌细胞株具有微摩尔范围的抗癌活性。通过研究取代基对其抗增殖活性的影响,进行了结构-活性关系(SAR)分析。其中一个在苯环的 R1、R2 和 R3 位置上带有甲氧基取代基的化合物 3i 显示出与参考药物顺铂相当的效力,而且对乳腺癌细胞株的选择性比 184B5 细胞高出两倍。DOI:10.2174/157018011794839475
文献信息
-
3-Substituted-4-hydroxycoumarin as a new scaffold with potent CDK inhibition and promising anticancer effect: Synthesis, molecular modeling and QSAR studies作者:Nehad A. Abdel Latif、Rasha Z. Batran、Mohammed A. Khedr、Mohamed M. AbdallaDOI:10.1016/j.bioorg.2016.06.005日期:2016.8A new series of 3-substituted-4-hydroxycoumarin derivatives was designed, synthesized, and evaluated for CDK inhibiting and anticancer activities. All the synthesized target compounds showed remarkably high affinity and selectivity towards CDK1B, compared to flavopiridol, with Ki values in the low nanomolar range (Ki=0.35-0.88nM). Most of them elicited considerable inhibiting effect against CDK9T1设计,合成并评估了一系列新的3-取代-4-羟基香豆素衍生物的CDK抑制和抗癌活性。与flavopiridol相比,所有合成的目标化合物均对CDK1B表现出极高的亲和力和选择性,Ki值在低纳摩尔范围内(Ki = 0.35-0.88nM)。它们中的大多数引起对CDK9T1的显着抑制作用(Ki = 3.26-23.45nM)。此外,所有目标化合物均针对18种类型的人类肿瘤细胞系进行了体外测试。hydr 3a,N-苯基吡唑啉衍生物6b和2-氨基吡啶基-3-甲腈衍生物8c是针对MCF-7乳腺癌细胞系的最有效抗癌剂(分别为IC50 = 0.21、0.21和0.23nM)。目标化合物3a,在MCF-7乳腺癌小鼠异种移植模型中进一步评估了6b和8c,并显示了10mg / kg剂量的体内功效。对接研究证实了CDK1B活性位点的独特结合模式,其得分高于黄酮哌啶醇。定量结构活动关系研究已经完成,揭示了0.81的高度预测力R(2)。
-
Design, synthesis and biological evaluation of some novel 3-cinnamoyl-4-hydroxy-2H-chromen-2-ones as antimalarial agents作者:Kuldeep Patel、Chandrabose Karthikeyan、N. S. Hari Narayana Moorthy、Girdhar Singh Deora、Viswas Raja Solomon、Hoyun Lee、Piyush TrivediDOI:10.1007/s00044-011-9694-1日期:2012.8A novel series of 3-cinnamoyl-4-hydroxy-2H-chromen-2-ones were designed, synthesized and screened for antiplasmodial activity. Eleven compounds of the series exhibited micromolar potency against chloroquine sensitive and chloroquine resistant strains. The most potent compound 4-hydroxy-3-(3-(4-nitrophenyl)acryloyl)-2H-chromen-2-one showed inhibitory potency (IC50) of 3.1 and 4 mu g/ml against chloroquine sensitive and chloroquine resistant strains, respectively. A structure activity relationship study was performed by correlating the effect of substituents with the antimalarial activity of the title compounds. The novel 3-cinnamoyl-4-hydroxy-2H-chromen-2-ones reported here should be good lead for further development of antimalarial agents that can overcome resistance.
表征谱图
-
氢谱1HNMR
-
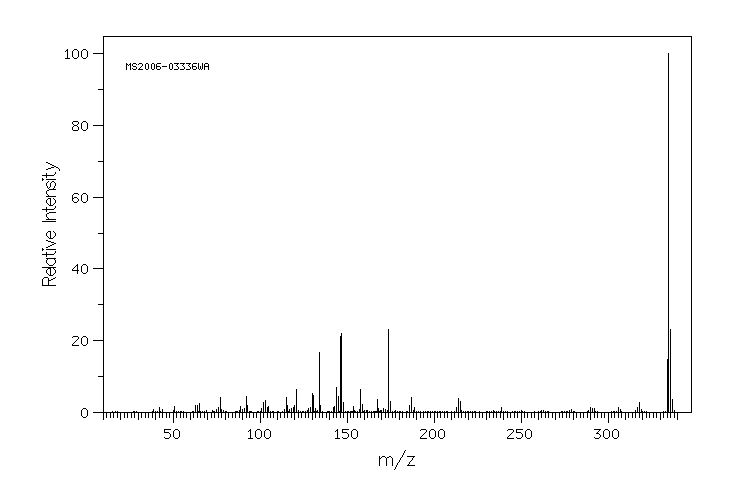
质谱MS
-
碳谱13CNMR
-
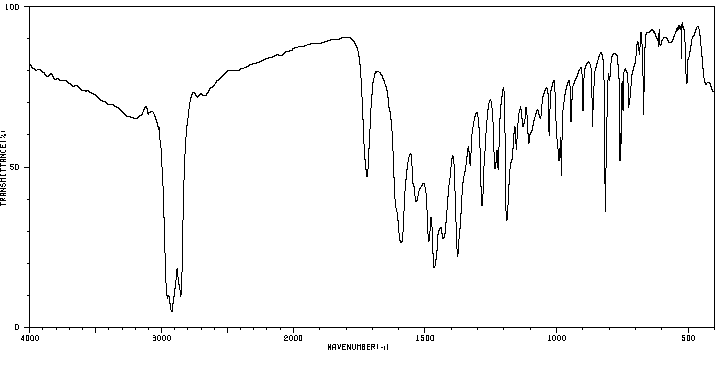
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯