N-Acetyl-L-valinol | 35593-71-6
中文名称
——
中文别名
——
英文名称
N-Acetyl-L-valinol
英文别名
N-[(2S)-1-hydroxy-3-methylbutan-2-yl]acetamide
CAS
35593-71-6
化学式
C7H15NO2
mdl
——
分子量
145.202
InChiKey
ILDSDBHYULTMIZ-SSDOTTSWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:306.8±25.0 °C(Predicted)
-
密度:0.979±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:49.3
-
氢给体数:2
-
氢受体数:2
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (S)-N-ethylvalinol 140424-69-7 C7H17NO 131.218
反应信息
-
作为反应物:描述:N-Acetyl-L-valinol 在 lithium aluminium tetrahydride 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 反应 19.0h, 生成 (2S,4S)-o-<2-(N-ethyl-4-isopropyl-1,3-oxazolidinyl)>benzaldehyde参考文献:名称:Highly Diastereoselective Reaction of Chiral o-(2-(1,3-Oxazolidinyl))benzaldehydes with Alkylmetallic Reagents: Synthesis of Chiral 3-Substituted Phthalides.摘要:高度非对映选择性的手性o-[2-(1,3-噁唑啉基)]苯甲醛(4-6)与烷基金属试剂反应,为手性3-烷基酞内酯提供了一种新颖的合成方法。DOI:10.1248/cpb.39.3136
-
作为产物:描述:(S)-2-(N-acetylamino)-3,3-dimethylpropanoic acid ethyl ester 在 sodium tetrahydroborate 作用下, 以 乙醇 为溶剂, 生成 N-Acetyl-L-valinol参考文献:名称:Seki,H. et al., Chemical and pharmaceutical bulletin, 1972, vol. 20, p. 361 - 367摘要:DOI:
文献信息
-
An Eco-friendly and Highly Efficient route for N-acylation under Catalyst-free Conditions作者:Souad Ouarna、Hacène K’tir、Salah Lakrout、Hamida Ghorab、Aïcha Amira、Zineb Aouf、Malika Berredjem、Nour AoufDOI:10.13005/ojc/310235日期:2015.6.20An eco-friendly, simple, mild, chemo selective and highly efficient procedure for the acylation of primary and secondary amine function in various structurally and electronically aliphatic and aromatic compounds affording their corresponding N-Ac derivatives is developed. Mild conditions, simplicity and easier work-up are the main advantages of this method.
-
Nanomole-Scale Assignment of Configuration for Primary Amines Using a Kinetic Resolution Strategy作者:Shawn M. Miller、Renzo A. Samame、Scott D. RychnovskyDOI:10.1021/ja310620c日期:2012.12.19The absolute configurations of primary amines were assigned using a kinetic resolution strategy with Mioskowski's enantioselective 1-(R,R) and 2-(S,S) acylating agents. A simple mnemonic was developed to determine the configuration. A pseudoenantiomeric pair of reagents, 1-(R,R) and 2-(S,S)-d(3), was prepared and used to assay primary amines on a micromolar scale. The ESI-MS readout of the resulting
-
[EN] TRICYCLIC HETEROCYCLES USEFUL AS TEAD BINDERS<br/>[FR] HÉTÉROCYCLES TRICYCLIQUES UTILES EN TANT QUE LIANTS TEAD申请人:MERCK PATENT GMBH公开号:WO2021224291A1公开(公告)日:2021-11-11The present invention relates to tricyclic heterocycles. These heterocyclic compounds are useful as TEAD binders and/or inhibitors of YAP-TEAD and TAZ-TEAD protein-protein interaction or binding and for the prevention and/or treatment of several medical conditions including hyperproliferative disorders and diseases, in particular cancer.
-
US7507820B2申请人:——公开号:US7507820B2公开(公告)日:2009-03-24
表征谱图
-
氢谱1HNMR
-
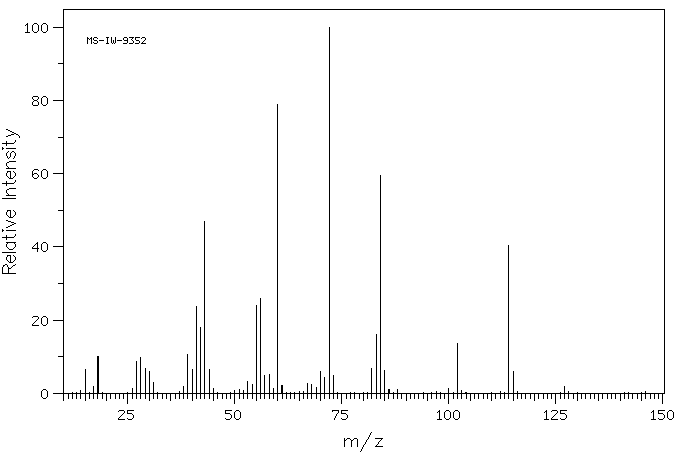
质谱MS
-
碳谱13CNMR
-
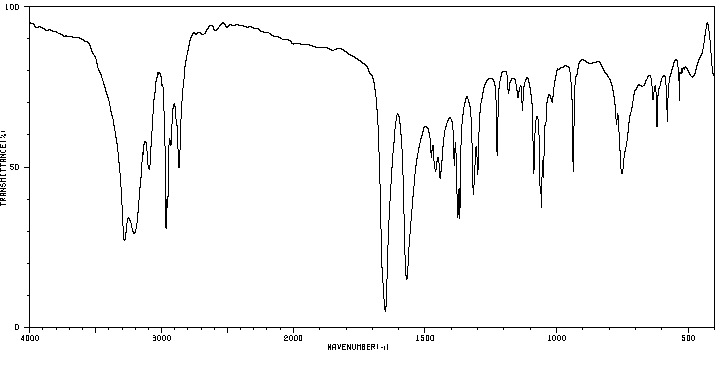
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸