3-(甲基硫代)异氰酸苯酯 | 28479-19-8
中文名称
3-(甲基硫代)异氰酸苯酯
中文别名
3-(甲基硫代)苯基异氰酸酯;3-(甲硫基)苯基异氰酸酯
英文名称
3-(methylthio)phenyl isocyanate
英文别名
(3-isocyanatophenyl)(methyl)sulfane;1-isocyanato-3-(methylsulfanyl)benzene;1-isocyanato-3-methylsulfanylbenzene
CAS
28479-19-8
化学式
C8H7NOS
mdl
MFCD00013863
分子量
165.216
InChiKey
BKJABLMNBSVKCV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:130 °C5 mm Hg(lit.)
-
密度:1.199 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解,未有已知危险反应。避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:54.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S23,S26,S36
-
危险类别码:R20/22,R36/37/38,R42
-
WGK Germany:3
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:UN 2922 8/PG 2
-
储存条件:冷藏保存
SDS
| Name: | 3-(Methylthio)phenyl isocyanate 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 28479-19-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 28479-19-8 | 3-(Methylthio)phenyl isocyanate | 97% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.Air sensitive.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Store under nitrogen.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 28479-19-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 129 - 130 deg C @5mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.199
Molecular Formula: C8H7NOS
Molecular Weight: 165
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, exposure to air.
Incompatibilities with Other Materials:
Oxidizing agents, reducing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, oxides of sulfur, carbon dioxide, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 28479-19-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-(Methylthio)phenyl isocyanate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: ISOCYANATE SOLUTION, TOXIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2206
Packing Group: III
IMO
Shipping Name: ISOCYANATE SOLUTION, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 2206
Packing Group: III
RID/ADR
Shipping Name: ISOCYANATE SOLUTION, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 2206
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 28479-19-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 28479-19-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 28479-19-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氨基茴香硫醚 3-(Methylthio)aniline 1783-81-9 C7H9NS 139.221
反应信息
-
作为反应物:描述:参考文献:名称:Pyrrolidylidene, piperidylidene and hexahydroazepinylidene ureas as CNS摘要:1-芳基-3-吡咯烷基脲类化合物,1-芳基-3-哌啶基脲类化合物和1-芳基-3-环己烷基脲类化合物属于中枢神经系统(CNS)抑制剂,具有实用价值。公开号:US03998958A1
-
作为产物:描述:参考文献:名称:Pyrrolidylidene, piperidylidene and hexahydroazepinylidene ureas as CNS摘要:1-芳基-3-吡咯烷基脲类化合物,1-芳基-3-哌啶基脲类化合物和1-芳基-3-环己烷基脲类化合物属于中枢神经系统(CNS)抑制剂,具有实用价值。公开号:US03998958A1
文献信息
-
[EN] NOVEL BENZAMIDE COMPOUNDS FOR USE IN MCH RECEPTOR RELATED DISORDERS<br/>[FR] NOUVEAUX COMPOSES DE BENZAMIDE DESTINES A ETRE UTILISES DANS DES TROUBLES ASSOCIES AU RECEPTEUR DE MCH申请人:7TM PHARMA AS公开号:WO2004048319A1公开(公告)日:2004-06-10Novel compounds of Formula I which modulate MCH activity are disclosed, in which A is a linker, Ar, is an aryl or heteroaryl group; R1 is hydrogen or a lower alkoxy group; Q together with the carbonyl forms an amide group, which is further substituted with an amine group; R5 is hydrogen, halogen atoms, alkoxy groups, hydroxy, alkylamino groups, dialkylamino groups, hydroxylalkyl groups, carboxamido groups, acylamido groups, acyl groups, -CHO, nitrile, alkyl, alkenyl or alkynyl groups, -SCH3, partially or fully fluorinated alkyl, alkoxy or thioalkoxy groups such as -CH2CF3, -CF2CF3, -CF3, -OCF3, -SCF3; -SO2NH2, -SO2NHAlk, -SO2NAlk2, -SO2AIk; R8 is hydrogen, halogen atoms, alkyl, alkenyl or alkynyl groups, cycloalkyl groups, alkylcycloalkyl groups, alkoxy groups, dialkylamino groups, -CONHAIk, -CONAIk2, - NHCO-Alk, -CO-Alk, -SCH3, partially or fully fluorinated alkyl, alkoxy or thioalkoxy groups such as -CH2CF3, -CF2CF3, -CF3, -OCF3, -SCF3; X is H, F, Cl, Br, I, -SCH3, partially or fully fluorinated alkyl, alkoxy or thioalkoxy groups such as -CH2CF3, - CF2CF3, -CF3, -OCF3, -SCF3; OCH3 or lower alkyl or alkenyl group; and which are useful in the treatment or prevention of e.g. obesity, depression, diabetes, bulimia etc.公开了调节MCH活性的Formula I的新化合物,其中A是连接剂,Ar是芳基或杂环芳基;R1是氢或较低的烷氧基;Q与羰基一起形成酰胺基团,该基团进一步被氨基取代;R5是氢、卤素原子、烷氧基、羟基、烷基氨基基团、二烷基氨基基团、羟基烷基基团、羧酰胺基团、酰胺基团、酰基、-CHO、腈基、烷基、烯基或炔基、-SCH3、部分或完全氟代烷基、烷氧基或硫代烷氧基基团,如-CH2 、-CF2CF3、- 、-O 、-S ;-SO2NH2、-SO2NHAlk、-SO2NAlk2、-SO2AIk;R8是氢、卤素原子、烷基、烯基或炔基、环烷基、烷基环烷基、烷氧基、二烷基氨基基团、-CONHAIk、-CONAIk2、-NHCO-Alk、-CO-Alk、-SCH3、部分或完全氟代烷基、烷氧基或硫代烷氧基基团,如-CH2 、-CF2 、- 、-O 、-S ;X是H、F、Cl、Br、I、-SCH3、部分或完全氟代烷基、烷氧基或硫代烷氧基基团,如-CH2 、-CF2 、- 、-O 、-S ;OCH3或较低的烷基或烯基基团;这些化合物在治疗或预防肥胖、抑郁症、糖尿病、暴食症等方面是有用的。
-
An automated, polymer-assisted strategy for the preparation of urea and thiourea derivatives of 15-membered azalides as potential antimalarial chemotherapeutics作者:Antun Hutinec、Renata Rupčić、Dinko Žiher、Kirsten S. Smith、Wilbur Milhous、William Ellis、Colin Ohrt、Zrinka Ivezić SchönfeldDOI:10.1016/j.bmc.2011.01.030日期:2011.3series of 15-membered azalide urea and thiourea derivatives has been synthesized and evaluated for their in vitro antimalarial activity against chloroquine-sensitive (D6), chloroquine/pyremethamine resistant (W2) and multidrug resistant (TM91C235) strains of Plasmodium falciparum. We have developed an effective automated synthetic strategy for the rapid synthesis of urea/thiourea libraries of a macrolide
-
PHENYL ISOCYANATE-BASED URETHANE ACRYLATES, PROCESSES FOR PRODUCING AND METHODS OF USING THE SAME申请人:Roelle Thomas公开号:US20100036013A1公开(公告)日:2010-02-11Urethane acrylates of the general Formula (I), corresponding salts, solvates or solvates of a salt thereof: wherein R 1 , R 2 , R 3 , R 4 and R 5 each independently represent a substituent selected from the group consisting of hydrogen, halogens, C 1-6 -alkyls, trifluoromethyl, C 1-6 -alkylthios, C 1-6 -alkylselenos, C 1-6 -alkyltelluros, and nitro groups, with the proviso that at least one of R 1 , R 2 , R 3 , R 4 and R 5 is not hydrogen; R 6 and R 7 each independently represent a substituent selected from the group consisting of hydrogen and C 1-6 -alkyls; and A represents a saturated or unsaturated or linear or branched C 1-6 -alkyl radical or a polyalkylene oxide radical having 2-6 ethylene oxide or propylene oxide units; processes for producing and methods of using the same.
-
NOVEL N-SUBSTITUTED-PYRROLIDINES AS INHIBITORS OF MDM2-P-53 INTERACTIONS申请人:Bartkovitz David Joseph公开号:US20110086854A1公开(公告)日:2011-04-14There are provided compounds of the formula wherein X, Y, R 1 , R 2 , R 3 , R 3 , R 4 , and R 5 are as described herein and enantiomers and pharmaceutically acceptable salts and esters thereof which are useful as anticancer agents.提供的化合物具有如下公式:其中X、Y、R1、R2、R3、R3、R4和R5如本文所述,以及作为抗癌剂有用的对映异构体和药用可接受的盐和酯。
-
Glucagon antagonists/inverse agonists申请人:Noro Nordisk A/S公开号:US06503949B1公开(公告)日:2003-01-07Disclosed is a novel class of compounds of formula (I) wherein V, A, Y, Z, R1, E, X and D are as defined in the specification. These compounds act to antagonize the action of the glucagon hormone on the glucagon receptor. Owing to their antagonizing effect of the glucagon receptor, the compounds are suitable for treating or preventing glucagon-mediated conditions and diseases such as hyperglycemia, Type 1 diabetes, Type 2 diabetes and obesity.揭示了一类新的化合物,其化学式为(I),其中V、A、Y、Z、R1、E、X和D的定义如规范中所述。这些化合物作用于拮抗胰高血糖素激素对胰高血糖素受体的作用。由于这些化合物对胰高血糖素受体的拮抗作用,它们适用于治疗或预防由胰高血糖素介导的疾病和病症,如高血糖、1型糖尿病、2型糖尿病和肥胖症。
表征谱图
-
氢谱1HNMR
-
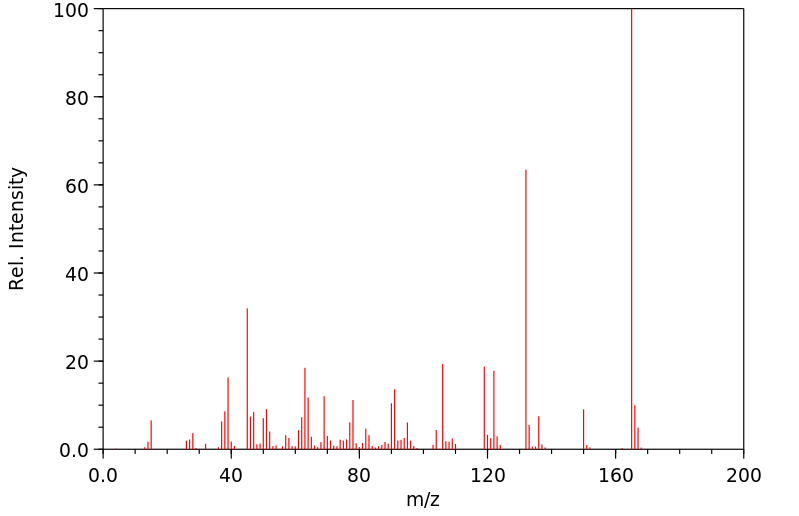
质谱MS
-
碳谱13CNMR
-
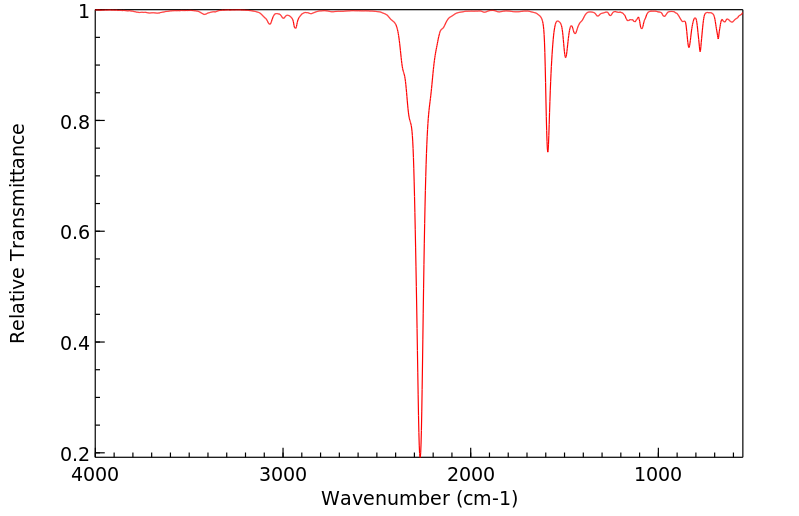
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯