代谢
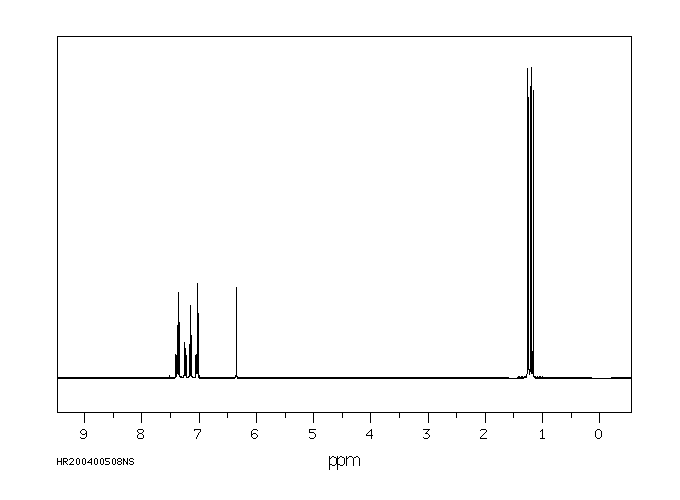
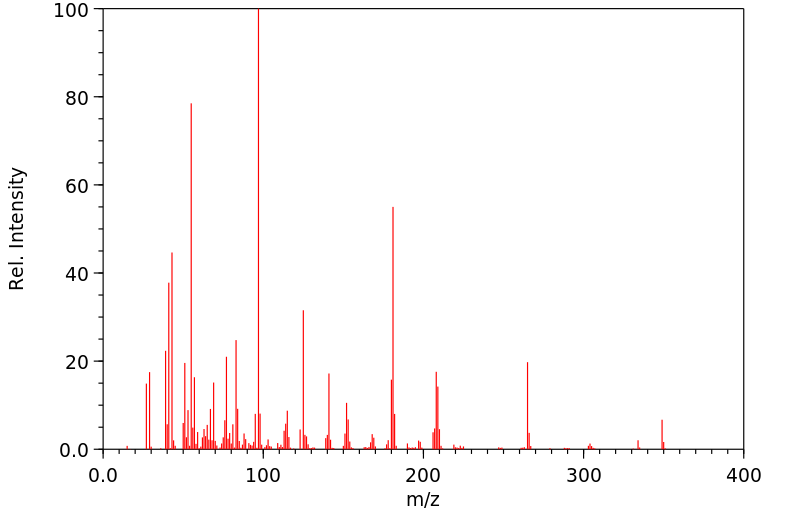
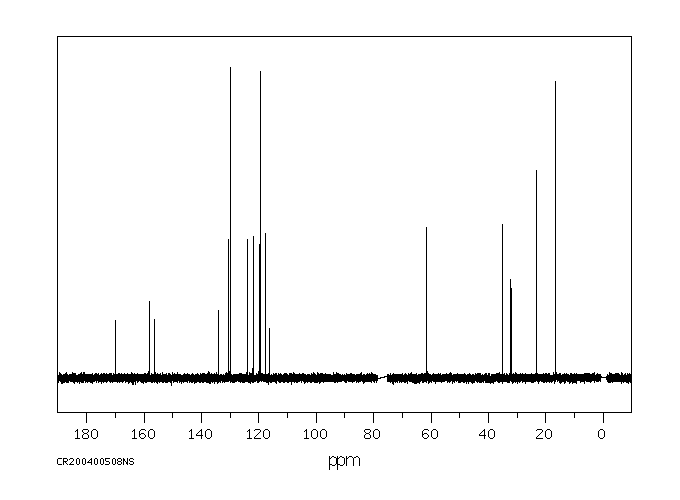
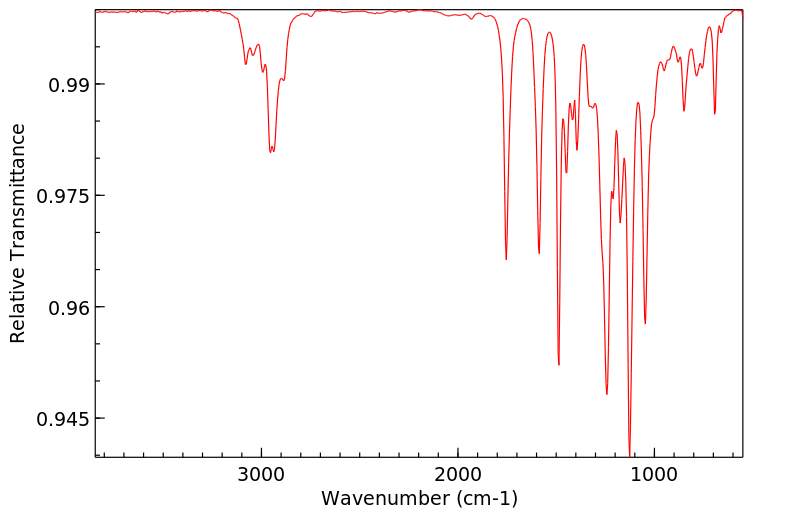
fenproponate在大鼠体内的代谢涉及迅速的氧化酶催化的酯裂解,随后是羟基化。
Metabolism of fenproponate in rats involves rapid oxidase catalyzed ester cleavage followed by hydroxylation.
来源:Hazardous Substances Data Bank (HSDB)